-
PDF
- Split View
-
Views
-
Cite
Cite
Toshiya Kurotobi, Katsuomi Iwakura, Koichi Inoue, Ryusuke Kimura, Yuko Toyoshima, Norihisa Ito, Hiroya Mizuno, Yoshihisa Shimada, Kenshi Fujii, Shinsuke Nanto, Issei Komuro, The significance of the shape of the left atrial roof as a novel index for determining the electrophysiological and structural characteristics in patients with atrial fibrillation, EP Europace, Volume 13, Issue 6, June 2011, Pages 803–808, https://doi.org/10.1093/europace/eur039
Close - Share Icon Share
Abstract
The silhouette of the left atrial (LA) roof is characterized by the pulmonary veins (PVs) and left atrium, and may include the characteristics of the PV/left atrium arrhythmogenicity in patients with atrial fibrillation (AF). In this study, we examined the hypothesis that the characteristics of the LA roof could help us understand the electrophysiological information of the PVs/left atrium.
The study population consisted of 153 consecutive patients with AF. The shape of the LA roof was determined by simultaneous right- and left-pulmonary angiography and 64-slice multi-detector-row computed tomography. The silhouette was classified into a deep-V shape (A; n= 35), shallow-V (B; n= 76) shape, and flat or coved shape (C; n= 42) according to the PV/left atrium dominancy. The AF triggers from the PVs (A: 70% vs. B: 57% vs. C: 40%; P= 0.003) became significantly less and those from non-PV sites (A: 6% vs. B: 13% vs. C: 22%; P= 0.043) significantly greater, as the LA shape became flat. Burst-inducible atrial tachyarrhythmias after PV isolation (A: 51% vs. B: 65% vs. C: 79%; P= 0.001) and at the end of the catheter ablation (A: 12% vs. B: 24% vs. C: 36%, P= 0.016) significantly increased, as the LA roof shape became flat. Although the PV diameters did not differ among the three groups, the LA volume (A: 69.5 ± 24.1 vs. B: 85.2 ± 34.9 vs. C: 105.7 ± 45.4 mL; P< 0.001) significantly increased as the LA shape became flat.
The shape of the LA roof allowed us to understand the structural and the electrophysiological information of the PVs/left atrium.
Introduction
Atrial fibrillation (AF) is mainly initiated by triggers from the pulmonary veins (PVs),1,2 and the pre-existent morphology of the PVs could modify their arrhythmogenicity.2–4 The development of the remodelling process could contribute to the structural and electrophysiological changes in the PVs and atrium; which could promote local conduction abnormalities and cause an increased PV/non-PV arrhythmogenicity resulting in AF persistency.3,5–8 This evidence supports the observation that the morphological findings of the PVs and atrium may play a crucial role of helping to identify the characteristics of their pre-existing arrhythmogenicity. However, there is a methodological limitation in using the PVs and atrium in order to evaluate the morphological characteristics in a quantitative manner because of their own unique, variable, and asymmetrical features.
The part of the left atrial (LA) roof that consists of the upper wall of the left atrium and upper PVs, incorporating the left atrium, was described as the LA roof silhouette, and could easily be visualized by pulmonary angiography or multi-detector-row computed tomography (MDCT) imaging. Further, we could easily define the morphological PVs/left atrium dominancy and the characteristics of the LA roof silhouette in patients with AF. In this study, we examined the hypothesis that the LA roof shape could be used as a novel predictor in patients with AF to allow us to determine the characteristics of the PVs, atrial arrhythmogenicity, and substrate for promoting AF.
Methods
Study population
The study population consisted of 153 consecutive patients with drug-refractory episodes of AF who underwent radiofrequency (RF) catheter ablation (CA). The patients’ mean age was 62 years, 122 (80%) were males, and 58 (38%) had persistent AF defined as recurrent episodes of AF lasting >3 months. The exclusion criteria were as follows: (i) a LA diameter of >55 mm, (ii) significant valvular disease requiring surgery, (iii) an ejection fraction of <40%, and (iv) hypertrophic obstructive cardiomyopathy. All anti-arrhythmic agents (AAAs) were generally discontinued for at least 3 days before the CA. Amiodarone was withdrawn at least 2 months before the procedure. All the patients provided written-informed consent for the electrophysiological study.
Electrophysiological study and catheter ablation
Transoesophageal echocardiography was performed to exclude any LA thrombi. A 10-pole or 20-polar diagnostic catheter was positioned in the coronary sinus (CS) for pacing and recording. A 20-pole catheter was located in the right atrium to cover the area around the tricuspid annulus or superior vena cava (SVC). The left atrium and PVs were accessed by a transseptal approach. We introduced three steerable catheters including two spiral curve catheters into the left atrium through a single transseptal puncture site. The PVs were mapped with a circumferential 10-pole or 20-pole catheter (IBI, Irvine, CA, USA). The surface electrocardiogram (ECG) and intracardiac electrograms filtered between 30 and 500 Hz were recorded simultaneously with a polygraph (DUO EP Laboratory; Bard Electrophysiology, Lowell, MA, USA). A single bolus of 150 IU/kg of heparin was administered after the transseptal puncture and repeated to maintain an activated clotting time of >300 s.
We initially performed a PV isolation procedure by using a double circular mapping technique during an isopreterenol administration (1–2 μg/min). We confirmed the success of the electrical PV isolation by monitoring the circumferential electrical isolation at the antrum level: ∼1 cm from the ostium of both the right and left PVs. The complete disappearance of the potentials from all four PVs was confirmed in all patients. In the patients with AF persistency even after PV isolation, direct cardioversion was initially attempted to restore sinus rhythm. After the PV isolation procedure, atrial tachyarrhythmias were induced by intense burst pacing. Atrial burst pacing was performed (10 s bursts) in decrements from 250 ms down to refractoriness at the maximum output or 150 ms from at least three sites including the distal CS and right atrium. Inducible AF was defined as sustained AF lasting for ≥1 min. In case of burst-inducible AF after the PV isolation procedure, an additional roof line was initially created. Then, the further additional RF energy applications were applied for mitral isthmus in cases with induced atrial tachycardia circuits and the still inducible AF. If the arrhythmogenic foci were suspected to have originated from a non-PV area, they were located by searching with a roving catheter.
Radiofrequency energy was delivered for 30–60 s at each site using an 8 mm tip catheter (Japan Life Line Co., Ltd, Fantasista, Tokyo, Japan). The RF energy was delivered with the power limited to 35 W. The temperature was limited to 55°C.
The induction and detection of the arrhythmogenic foci
The induction of arrhythmogenic foci was performed according to our previously reported paper.9 In brief, spontaneous arrhythmogenic foci in both atria were induced and carefully mapped before and after the PV isolation procedure using an intravenous infusion of high-dose isoproterenol (ISP) of up to 20 μg/min without any sedation. If AF persisted or spontaneously occurred under the ISP, we attempted to cardiovert the AF up to three times. To detect the location of the arrhythmogenic foci, we simultaneously used five multipolar catheters to record the electrograms from the PVs and outside the PVs to search for any arrhythmogenic foci. A 20-pole catheter (2 mm inter-electrode spacing) covered the area from the SVC to the crista terminalis, CS, and ostium of the left PVs. A roving catheter was located at the right superior PV ostium. During the ablation procedure, the ISP administration was maintained at 1–2 μg/min. At the end of the procedure, the same induction manoeuvres as in the initial protocol (up to 20 μg/min) were repeated. Arrhythmogenic foci were defined as direct AF triggers or spontaneous reproducible atrial premature beats with coupling intervals of <350 ms or frequent repetitive firings.
The evaluation of the cardiac parameters
We measured the end-systolic LA diameter and left ventricular parameters with 2D echocardiography. The LA volume and PV diameter were measured by integrating the volume traced in each slice of the 64-slice MDCT scan (Philips Medicals Systems, Haifa, Israel) during several days prior to the CA. To enhance the cardiac cavity, contrast medium was injected at a flow rate of 2.5 mL/s through an antecubital vein using an injector. The LA volume was measured by integrating the volume traced in each slice of the CT scan from the level of the mitral annulus to the roof of the left atrium with commercially available software (EP planner, Philips Medical Systems, Haifa, Israel). Each slice was automatically traced with digital markers to exclude the PVs and LA appendage at their ostial level. The LA appendage was excluded from the volumetric analysis.
The assessment of the left atrial roof shape
According to the PVs and LA-dominant level, we classified the LA roof shape into a deep V shape (Group A; possible PV-dominant type), shallow V shape (Group B), and flat-coved shape (Group C; possible LA-dominant type) using both PVs cine angiography (Figure 1). Cine angiography was performed by spontaneous contrast medium injection from the long sheath located at the upper right and left PVs. The shape of LA roof was determined by using antero-posterior projection, and was assessed by an upper angle between the right- and left-side LA wall silhouette. Deep V shape (A) was defined at an <140°, shallow, V shape (B) was 140–180°, and flat-coved shape was >180° (C).
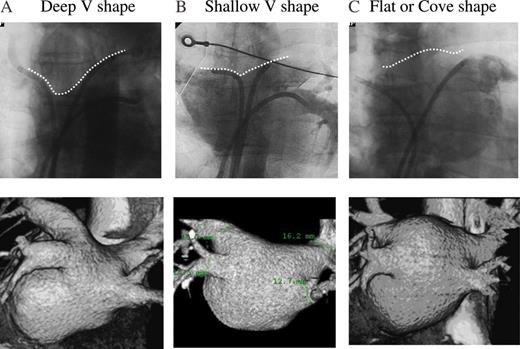
According to the pulmonary vein and left atrial-dominant level, we classified the left atrial roof shape into a deep V shape (A), shallow V shape (B), and flat-coved shape (C) by using cine angiography and 64-slice multi-detector-row computed tomography. The upper panels show the cineangiography, and the lower panels the 3D constructed image of the 64-slice multi-detector-row computed tomography. The deep V shape was mainly influenced by the segment of both trunks of the upper pulmonary veins, whereas the pulmonary veins were less incorporated into the left atrial with the flat-coved shape.
Follow-up
All patients were discharged home 3 days after the CA procedure and were seen in our hospital at 1–2-month intervals. The in-hospital AF episodes were carefully monitored for at least 2 days after the CA, and the AF episodes after discharge were adequately assessed by the patients’ complaints, 12-lead ECG and 24 h Holter ECG recordings. Atrial fibrillation recurrence was defined as the occurrence of atrial tachyarrhythmias after a 2-month blanking period following the CA procedure. All anti-arrhythmic agents were given for 3–6 months to the patients with long-lasting persistent AF or to those with paroxysmal AF and easily induced residual AF. Following that, the anti-arrhythmic drugs (AADs) were withdrawn and the AF episodes were further assessed without AADs.
Statistical analysis
The continuous variables with a normal distribution were expressed as the mean ± SD. The percentage of the variables or the distribution was compared by a χ2 test. The variables without a normal distribution were compared by a Mann–Whitney U test that was used for the non-parametric analysis. The analyses of the trends for the each roof shape were performed with a one-way analysis of variance (ANOVA). A logistic multivariate analysis was used to assess the relationship between the predictor variables and AF trigger from PVs, and backward selection using P< 0.05 (Wald test) to stay was used to detect significant independent factors related to AF trigger from PVs. The possible confounding clinical factors (age, male gender, structural heart disease, hypertension, AF duration, number of AADs used, and LA volume) related to the development of LA roof V shape and AF triggers from PVs were incorporated into the multivariate model. The analyses of the trends for the each roof shape were performed with a one-way ANOVA. All analyses were performed using SPSS 10. 0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
The comparison of the patient characteristics among Groups A–C are shown in Table 1. Group A was observed in 35 patients (23%), B in 76 (50%), and C in 42 patients (27%). There were no significant differences in the mean age, mean AF duration, or clinical coexistence of atrial flutter, among the three groups. As the LA roof silhouette became flat, the number of prior of AADs (A: 2.1 ± 0.9 vs. B: 1.7 ± 1.4 vs. C: 1.2 ± 0.8; P= 0.003) significantly decreased, and the prevalence of structural heart disease (A: 19% vs. B: 40% vs. C; 68%; P= 0.002), and persistent AF (A: 26% vs. B: 35% vs. C: 52%; P= 0.014) significantly increased.
| . | Group A . | Group B . | Group C . | P value . |
|---|---|---|---|---|
| . | (n= 35) . | (n= 76) . | (n= 42) . | . |
| Age (years) | 64.7 ± 6.9 | 61.4 ± 11.3 | 62.6 ± 8.7 | 0.40 |
| Male (%) | 83 | 75 | 86 | 0.69 |
| SHD (%) | 19 | 40 | 68 | 0.002 |
| Hypertension (%) | 30 | 24 | 46 | 0.10 |
| AF period (m) | 63.8 | 67.1 | 67.0 | 0.83 |
| Per-AF | 26 | 35 | 52 | 0.014 |
| The duration of pe-AF | 14.3 | 22.7 | 37.8 | 0.108 |
| Co-AFL (%) | 27 | 26 | 39 | 0.42 |
| # Of AAD | 2.1 ± 0.9 | 1.7 ± 1.4 | 1.2 ± 0.8 | 0.003 |
| . | Group A . | Group B . | Group C . | P value . |
|---|---|---|---|---|
| . | (n= 35) . | (n= 76) . | (n= 42) . | . |
| Age (years) | 64.7 ± 6.9 | 61.4 ± 11.3 | 62.6 ± 8.7 | 0.40 |
| Male (%) | 83 | 75 | 86 | 0.69 |
| SHD (%) | 19 | 40 | 68 | 0.002 |
| Hypertension (%) | 30 | 24 | 46 | 0.10 |
| AF period (m) | 63.8 | 67.1 | 67.0 | 0.83 |
| Per-AF | 26 | 35 | 52 | 0.014 |
| The duration of pe-AF | 14.3 | 22.7 | 37.8 | 0.108 |
| Co-AFL (%) | 27 | 26 | 39 | 0.42 |
| # Of AAD | 2.1 ± 0.9 | 1.7 ± 1.4 | 1.2 ± 0.8 | 0.003 |
SHD, structural heart disease; Co-AFL, coexistent atrial flutter; AAD, anti-arrhythmic drug; per-AF, persistent AF.
| . | Group A . | Group B . | Group C . | P value . |
|---|---|---|---|---|
| . | (n= 35) . | (n= 76) . | (n= 42) . | . |
| Age (years) | 64.7 ± 6.9 | 61.4 ± 11.3 | 62.6 ± 8.7 | 0.40 |
| Male (%) | 83 | 75 | 86 | 0.69 |
| SHD (%) | 19 | 40 | 68 | 0.002 |
| Hypertension (%) | 30 | 24 | 46 | 0.10 |
| AF period (m) | 63.8 | 67.1 | 67.0 | 0.83 |
| Per-AF | 26 | 35 | 52 | 0.014 |
| The duration of pe-AF | 14.3 | 22.7 | 37.8 | 0.108 |
| Co-AFL (%) | 27 | 26 | 39 | 0.42 |
| # Of AAD | 2.1 ± 0.9 | 1.7 ± 1.4 | 1.2 ± 0.8 | 0.003 |
| . | Group A . | Group B . | Group C . | P value . |
|---|---|---|---|---|
| . | (n= 35) . | (n= 76) . | (n= 42) . | . |
| Age (years) | 64.7 ± 6.9 | 61.4 ± 11.3 | 62.6 ± 8.7 | 0.40 |
| Male (%) | 83 | 75 | 86 | 0.69 |
| SHD (%) | 19 | 40 | 68 | 0.002 |
| Hypertension (%) | 30 | 24 | 46 | 0.10 |
| AF period (m) | 63.8 | 67.1 | 67.0 | 0.83 |
| Per-AF | 26 | 35 | 52 | 0.014 |
| The duration of pe-AF | 14.3 | 22.7 | 37.8 | 0.108 |
| Co-AFL (%) | 27 | 26 | 39 | 0.42 |
| # Of AAD | 2.1 ± 0.9 | 1.7 ± 1.4 | 1.2 ± 0.8 | 0.003 |
SHD, structural heart disease; Co-AFL, coexistent atrial flutter; AAD, anti-arrhythmic drug; per-AF, persistent AF.
Relation between the left atrial roof shapes and electrophysiological characteristics
The electrophysiological characteristics of each roof shape group are shown in Table 2. Three hundred and thirty-five arrhythmogenic foci were found. Pulmonary vein and/or non-PV foci were revealed in 136 of 152 (89.4%) patients. Atrial fibrillation triggers directly shifting to AF were observed in 114 of 152 (75.0%) patients, and AF triggered by PV foci from four PVs was observed in 84 of 152 (55.2%) patients. Pulmonary vein foci including reproducible atrial premature beats were detected in 135 of 152 patients (88.8%), and non-PV foci in 77 of 152 (44%). The location of 90 non-PV foci included the LA posterior wall (19, 21.1%), SVC (25, 27.8%), crista terminalis (10, 11.1%), lateral mitral area (9, 10%), LA roof (7, 7.8%), inter-atrial septum (4, 4.4%), coronary sinus (10, 11.1%), and other sites (6, 6.7%).
| . | Group A . | Group B . | Group C . | P value . |
|---|---|---|---|---|
| . | (n= 35) . | (n= 76) . | (n= 42) . | . |
| Trigger inducible AF (%) | ||||
| AF from four PVs | 70 | 57 | 40 | 0.003 |
| AF from upper PVs | 63 | 41 | 38 | 0.046 |
| AF from non-PV sites | 6 | 13 | 22 | 0.041 |
| AFCs from four PVs | 94 | 84 | 76 | 0.033 |
| AFCs from upper PVs | 90 | 76 | 64 | 0.022 |
| AFCs from non-PV sites | 26 | 46 | 54 | 0.016 |
| # Of AF foci from PVs | 0.86 | 0.76 | 0.80 | 0.82 |
| # Of all AF foci | 2.03 | 1.93 | 2.29 | 0.28 |
| Pacing inducible AF (%) | ||||
| Just after PVI | 51 | 65 | 79 | 0.001 |
| At the end of the CA | 12 | 24 | 36 | 0.016 |
| . | Group A . | Group B . | Group C . | P value . |
|---|---|---|---|---|
| . | (n= 35) . | (n= 76) . | (n= 42) . | . |
| Trigger inducible AF (%) | ||||
| AF from four PVs | 70 | 57 | 40 | 0.003 |
| AF from upper PVs | 63 | 41 | 38 | 0.046 |
| AF from non-PV sites | 6 | 13 | 22 | 0.041 |
| AFCs from four PVs | 94 | 84 | 76 | 0.033 |
| AFCs from upper PVs | 90 | 76 | 64 | 0.022 |
| AFCs from non-PV sites | 26 | 46 | 54 | 0.016 |
| # Of AF foci from PVs | 0.86 | 0.76 | 0.80 | 0.82 |
| # Of all AF foci | 2.03 | 1.93 | 2.29 | 0.28 |
| Pacing inducible AF (%) | ||||
| Just after PVI | 51 | 65 | 79 | 0.001 |
| At the end of the CA | 12 | 24 | 36 | 0.016 |
LA, left atrial; PVs, pulmonary veins; PVI, pulmonary vein isolation; CA, catheter ablation procedure; AF, atrial fibrillation; AFCs, arrhythmigenic foci. AFCs include reproducible atrial premature beats as the possible AF trigger.
| . | Group A . | Group B . | Group C . | P value . |
|---|---|---|---|---|
| . | (n= 35) . | (n= 76) . | (n= 42) . | . |
| Trigger inducible AF (%) | ||||
| AF from four PVs | 70 | 57 | 40 | 0.003 |
| AF from upper PVs | 63 | 41 | 38 | 0.046 |
| AF from non-PV sites | 6 | 13 | 22 | 0.041 |
| AFCs from four PVs | 94 | 84 | 76 | 0.033 |
| AFCs from upper PVs | 90 | 76 | 64 | 0.022 |
| AFCs from non-PV sites | 26 | 46 | 54 | 0.016 |
| # Of AF foci from PVs | 0.86 | 0.76 | 0.80 | 0.82 |
| # Of all AF foci | 2.03 | 1.93 | 2.29 | 0.28 |
| Pacing inducible AF (%) | ||||
| Just after PVI | 51 | 65 | 79 | 0.001 |
| At the end of the CA | 12 | 24 | 36 | 0.016 |
| . | Group A . | Group B . | Group C . | P value . |
|---|---|---|---|---|
| . | (n= 35) . | (n= 76) . | (n= 42) . | . |
| Trigger inducible AF (%) | ||||
| AF from four PVs | 70 | 57 | 40 | 0.003 |
| AF from upper PVs | 63 | 41 | 38 | 0.046 |
| AF from non-PV sites | 6 | 13 | 22 | 0.041 |
| AFCs from four PVs | 94 | 84 | 76 | 0.033 |
| AFCs from upper PVs | 90 | 76 | 64 | 0.022 |
| AFCs from non-PV sites | 26 | 46 | 54 | 0.016 |
| # Of AF foci from PVs | 0.86 | 0.76 | 0.80 | 0.82 |
| # Of all AF foci | 2.03 | 1.93 | 2.29 | 0.28 |
| Pacing inducible AF (%) | ||||
| Just after PVI | 51 | 65 | 79 | 0.001 |
| At the end of the CA | 12 | 24 | 36 | 0.016 |
LA, left atrial; PVs, pulmonary veins; PVI, pulmonary vein isolation; CA, catheter ablation procedure; AF, atrial fibrillation; AFCs, arrhythmigenic foci. AFCs include reproducible atrial premature beats as the possible AF trigger.
As the LA roof silhouette became flat, the incidence of AF arising from the PVs (A: 70% vs. B: 57% vs. C: 40%; P= 0.003), AF from the upper PVs (A: 63% vs. B: 41% vs. C: 38%; P= 0.046), and from arrhythmogenic foci including reproducible premature beats (A: 94% vs. B: 84% vs. C: 76%; P= 0.033) significantly decreased. On the other hand, the incidence of AF arising from non-PV sites (A: 6% vs. B: 13% vs. C: 22%; P= 0.041) and from arrhythmogenic foci from non-PV sites (A: 26% vs. B: 46% vs. C: 54%; P= 0.016) significantly increased as the LA roof silhouette became flat. A multivariate analysis demonstrated that the deep V shape was an independent contributing factor to AF triggers from PV [odds ratio (95% confidence interval); 2.94 (1.27–6.80); P= 0.012].
The incidence of pacing inducible AF just after the PV isolation was observed in 100 of 152 (65.8%) patients, and spontaneous AF in 37 of 152 (24.3%) patients at the end of the CA. As the LA roof silhouette became flat, the incidence of pacing inducible AF just after the PV isolation (A: 51% vs. B: 65% vs. C: 79%; P= 0.001) and at the end of the CA (A: 12% vs. B: 24% vs. C: 36%; P= 0.016) significantly increased.
Relationship of the left atrial roof shapes and structural findings
The structural findings for each roof shape group are shown in Table 3. The LA diameter (A-P: 33.1 ± 2.8 mm for A vs. 37.4 ± 5.6 mm for B vs. 40.2 ± 6.3 mm for C, P< 0.001;. S-L: 36.1 ± 5.8 mm for A vs. 39.4 ± 6.6 mm for B vs. 43.2 ± 6.8 mm for C, P< 0.001; MV-PV: 48.2 ± 6.0 mm for A vs. 55.3 ± 8.7 mm for B vs. 58.4 ± 7.6 mm for C, P< 0.001.) and entire LA volume (A: 69.5 ± 24.1 mL vs. B: 85.2 ± 34.9 mL vs. C: 105.7 ± 45.4; P< 0.001) became significantly larger, as the LA silhouette became flat. The PV diameter of each PV and left ventricular ejection fraction did not differ between the three roof shape groups.
| . | Group A . | Group B . | Group C . | P value . |
|---|---|---|---|---|
| . | (n= 35) . | (n= 76) . | (n= 42) . | . |
| Left atrial diameter (mm) | ||||
| (A-P) | 33.1 ± 2.8 | 37.4 ± 5.6 | 40.2 ± 6.3 | 0.001 |
| (S-L) | 36.1 ± 5.8 | 39.4 ± 6.6 | 43.2 ± 6.8 | 0.001 |
| (MV-PV) | 48.2 ± 6.0 | 55.3 ± 8.7 | 58.4 ± 7.6 | 0.001 |
| LA volume (cm3) | 69.5 ± 24.1 | 85.2 ± 34.9 | 105.7 ± 45.4 | 0.001 |
| PV diameter (mm) | ||||
| (LSPV) | 18.5 ± 2.8 | 19.0 ± 2.9 | 19.5 ± 4.8 | 0.19 |
| (LIPV) | 15.3 ± 2.4 | 15.8 ± 2.2 | 15.5 ± 3.2 | 0.74 |
| (RSPV) | 18.6 ± 2.6 | 19 ± 3.2 | 19.3 ± 3.7 | 0.32 |
| (RIPV) | 15.8 ± 2.2 | 15.9 ± 2.6 | 15.7 ± 2.9 | 0.83 |
| LVEF (%) | 66.1 ± 8.6 | 63.3 ± 13.7 | 59.7 ± 13.1 | 0.08 |
| . | Group A . | Group B . | Group C . | P value . |
|---|---|---|---|---|
| . | (n= 35) . | (n= 76) . | (n= 42) . | . |
| Left atrial diameter (mm) | ||||
| (A-P) | 33.1 ± 2.8 | 37.4 ± 5.6 | 40.2 ± 6.3 | 0.001 |
| (S-L) | 36.1 ± 5.8 | 39.4 ± 6.6 | 43.2 ± 6.8 | 0.001 |
| (MV-PV) | 48.2 ± 6.0 | 55.3 ± 8.7 | 58.4 ± 7.6 | 0.001 |
| LA volume (cm3) | 69.5 ± 24.1 | 85.2 ± 34.9 | 105.7 ± 45.4 | 0.001 |
| PV diameter (mm) | ||||
| (LSPV) | 18.5 ± 2.8 | 19.0 ± 2.9 | 19.5 ± 4.8 | 0.19 |
| (LIPV) | 15.3 ± 2.4 | 15.8 ± 2.2 | 15.5 ± 3.2 | 0.74 |
| (RSPV) | 18.6 ± 2.6 | 19 ± 3.2 | 19.3 ± 3.7 | 0.32 |
| (RIPV) | 15.8 ± 2.2 | 15.9 ± 2.6 | 15.7 ± 2.9 | 0.83 |
| LVEF (%) | 66.1 ± 8.6 | 63.3 ± 13.7 | 59.7 ± 13.1 | 0.08 |
LA, left atrial.
| . | Group A . | Group B . | Group C . | P value . |
|---|---|---|---|---|
| . | (n= 35) . | (n= 76) . | (n= 42) . | . |
| Left atrial diameter (mm) | ||||
| (A-P) | 33.1 ± 2.8 | 37.4 ± 5.6 | 40.2 ± 6.3 | 0.001 |
| (S-L) | 36.1 ± 5.8 | 39.4 ± 6.6 | 43.2 ± 6.8 | 0.001 |
| (MV-PV) | 48.2 ± 6.0 | 55.3 ± 8.7 | 58.4 ± 7.6 | 0.001 |
| LA volume (cm3) | 69.5 ± 24.1 | 85.2 ± 34.9 | 105.7 ± 45.4 | 0.001 |
| PV diameter (mm) | ||||
| (LSPV) | 18.5 ± 2.8 | 19.0 ± 2.9 | 19.5 ± 4.8 | 0.19 |
| (LIPV) | 15.3 ± 2.4 | 15.8 ± 2.2 | 15.5 ± 3.2 | 0.74 |
| (RSPV) | 18.6 ± 2.6 | 19 ± 3.2 | 19.3 ± 3.7 | 0.32 |
| (RIPV) | 15.8 ± 2.2 | 15.9 ± 2.6 | 15.7 ± 2.9 | 0.83 |
| LVEF (%) | 66.1 ± 8.6 | 63.3 ± 13.7 | 59.7 ± 13.1 | 0.08 |
| . | Group A . | Group B . | Group C . | P value . |
|---|---|---|---|---|
| . | (n= 35) . | (n= 76) . | (n= 42) . | . |
| Left atrial diameter (mm) | ||||
| (A-P) | 33.1 ± 2.8 | 37.4 ± 5.6 | 40.2 ± 6.3 | 0.001 |
| (S-L) | 36.1 ± 5.8 | 39.4 ± 6.6 | 43.2 ± 6.8 | 0.001 |
| (MV-PV) | 48.2 ± 6.0 | 55.3 ± 8.7 | 58.4 ± 7.6 | 0.001 |
| LA volume (cm3) | 69.5 ± 24.1 | 85.2 ± 34.9 | 105.7 ± 45.4 | 0.001 |
| PV diameter (mm) | ||||
| (LSPV) | 18.5 ± 2.8 | 19.0 ± 2.9 | 19.5 ± 4.8 | 0.19 |
| (LIPV) | 15.3 ± 2.4 | 15.8 ± 2.2 | 15.5 ± 3.2 | 0.74 |
| (RSPV) | 18.6 ± 2.6 | 19 ± 3.2 | 19.3 ± 3.7 | 0.32 |
| (RIPV) | 15.8 ± 2.2 | 15.9 ± 2.6 | 15.7 ± 2.9 | 0.83 |
| LVEF (%) | 66.1 ± 8.6 | 63.3 ± 13.7 | 59.7 ± 13.1 | 0.08 |
LA, left atrial.
Clinical outcome
An in-hospital recurrence was observed in 28 of 152 (18%) patients, and a long-term AF recurrence was observed in 29 of 152 (19%) patients. The mean follow-up period after the CA was 567 days (360–1065 days). The AADs used were administrated after the CA in 30% of the patients (persistent: 47% and paroxysmal: 23%). The ratio of additional LA roof line (A: 64% vs. B: 84% vs. C: 88%; P< 0.05), and mitral isthmus line (A: 14% vs. B: 22% vs. C: 34%; P< 0.05) during CA were significantly highly required as the LA silhouette became flat, whereas the AF recurrence was not significantly different among three groups (A: 18% vs. B: 18% vs. C: 23%; n.s.).
Discussion
In this study, we classified the LA roof shapes into a deep V shape (PV dominancy), shallow V shape, and flat-coved shape (left atrium dominancy) in the AF patients according to the structural PVs/left atrium dominancy. For the clinical background, an increased prevalence of structural heart disease and persistent AF were significantly associated with a flat-coved roof shape; however, an increased number of prior AADs were significantly associated with a deep V shape. In the electrophysiological findings, an increased incidence of PV foci was associated with a deep V shape; however, an increased incidence of non-PV foci and burst-inducible AF just after the PV isolation or at the end of the CA were likely to be associated with a flat roof shape. The flat LA roof shape was mainly determined by an increased LA volume; which implied the development of a structural remodelling process that could promote a shift of the LA roof shape from a deep shape to a flat shape.
Left atrial roof shape and pulmonary vein/left atrium dominancy
From the embryological view, the PV trunks are shown to derive from a common vessel, which becomes absorbed within the left atrium from superior–posterior direction. This incorporation transforms the branches of this common PV into four individual, separately inserting PV trunks, first into the right and left PV trunks and subsequently into the superior and inferior trunks.10–12 Anatomical PV structure could be caused by either anomalous branching of the common PV or by the variable absorption level of the common PV into the left atrium, therefore the incorporation level could mainly determine the LA roof shape according to the PV/LA dominancy level. When the common PV was incorporated into the left atrium with the PVs having dominancy, the silhouette of the LA roof had a V shape. When the PV was incorporated with the atrial wall having dominancy, the LA roof tended to have a flat shape.
In this study, an enlarged LA volume has a significant association with flat or coved LA roof shape. The development of the atrial structural remodelling process may also change the LA roof shape. Vertical LA enlargement could modify LA roof shape and sometimes overlap the PV silhouette as a component of LA roof, because the location of PVs is strictly stick to right and left lungs. Horizontal LA enlargement may promote the development of flat LA roof. Moreover, the advancement of cardiac rotation as a result of ageing or hypertensive change; which is possibly related to atrial remodelling process, may also change the LA roof silhouette.
Left atrial roof shape and pulmonary vein arrhythmogenicity
Atrial fibrillation is mainly initiated by PV triggers,1 and a rapidly firing source located within the PVs could be responsible for initiating, and in some cases, maintaining arrhythmias in patients with AF. The mechanism underlying such rapid discharges from PVs, including enhanced automaticity or triggered activity mechanisms may be involved in the initiation of AF.13
In this study, AF triggers from PVs were highly observed in the patients with a deep V LA roof shape and PV dominancy. A previous paper reported that AF tended to originate from larger PVs.14 Especially in the superior PVs, the enlargement may often be consistent with the site of the arrhythmogenic PVs.2 Pulmonary vein enlargement caused by the stretch mechanism may increase the PV's automaticity and/or triggered activity to initiate AF.15 However, there was no significant relation between the LA roof shape and PV diameter in this study. These findings may imply the novelty and independence of the LA roof shape as an index of the PV's arrhythmogenicity, as compared with the prior reports that discussed the PV features.
Left atrial roof shape and non-pulmonary vein arrhythmogenicity
Non-PV foci could arise from the SVC, LA posterior free wall, crista terminalis, ostium of the coronary sinus, inter atrial septum, or Marshall bundle16,17 with an incidence of those ranging from 3.2 to 47%.18–20 The predominant non-PV triggering sites have a slow diastolic depolarization that enhances spontaneous depolarization,7 and the triggered activity of the non-PV triggers could also be involved in the onset and perpetuation of AF.
Because triggered activity is likely to occur in the presence of underlying disease such as cardiomyopathy,21 the development of the atrial remodelling process may enhance the triggered activity of non-PV lesions. A previous study reported that multiple PV arrhythmogenic foci may be associated with an older age, longer AF duration, and larger atrial dimensions.22 Left atrial enlargement predisposes the atrium to LA posterior wall triggers,3 and persistent AF is more frequently triggered by foci from the LA side of the LA–PV junction than is paroxysmal AF.4 These findings could explain why the flat and cove LA roof as a result of advanced atrial remodelling increased the non-PV arrhythmogenisity in this study.
The significance of left atrial roof shape as an ablation strategy
It is now recognized that the development of AF leads to electrical and structural changes within the atria that perpetuate the atrial tachyarrhythmia. The structural changes, including the enlarged left atrium, further promote the inconsistency and prolongation of the atrial conduction which leads to maintaining the perpetuation of AF. In this study, the incidence of pacing inducible AF just after PV isolation and at the end of the CA significantly increased, as the LA roof silhouette became flat. A previous paper reported that AF recurrence after CA was significantly higher in patients with inducible AF after the PV isolation than in those without AF,23 therefore that evidence indicates the importance of a flat or coved LA roof shape as a reflection of any latent AF substrate in the atrium. And also, the development of the atrial remodelling process could enhance the triggered activity of non-PV lesions.3,21 In this study, non-PV foci were significantly more often observed in patients with a flat-coved LA roof shape.
Pulmonary vein isolation is the cornerstone of the treatment especially in patients with paroxysmal AF; however, the PV electrical isolation strategy alone in patients with a remodelled atrium might be quite limited. Many of the AF recurrences are thought to be secondary to electrical and structural remodelling.5,24 Thus, a further additional extensive intervention following the PV isolation might be required to improve the outcome of the CA in patients with a flat-coved LA roof, whereas only a PV isolation strategy could lead to a favourable outcome in patients with a deep V LA roof.
Limitation
Atrial fibrillation induced by ISP or burst pacing manoeuvre may not have physiological methods as clinical AF index.
Clinical implications
The LA roof was a crucial and sensitive part for reflecting the extent of the PV/left atrium dominancy and remodelling; which provided us with an understanding of the arrhythmogenicity of the PVs and left atrium, and the substrate for promoting AF in patients with AF. This information is useful for determining the appropriate strategy for the CA of AF. The clinical importance of LA roof shape should be prospectively confirmed in same treatment AF strategy.
Conflict of interest: none declared.



