-
PDF
- Split View
-
Views
-
Cite
Cite
Francisco Buendía, Óscar Cano, Juan Miguel Sánchez-Gómez, Begoña Igual, Joaquín Osca, María José Sancho-Tello, José Olagüe, Antonio Salvador, Cardiac magnetic resonance imaging at 1.5 T in patients with cardiac rhythm devices, EP Europace, Volume 13, Issue 4, April 2011, Pages 533–538, https://doi.org/10.1093/europace/euq501
Close - Share Icon Share
Abstract
Recent studies suggest that non-cardiac magnetic resonance imaging (MRI) scanning can be performed safely in selected cardiac rhythm device patients. However, little is known about the safety of performing specific cardiac MRI in this setting. We sought to determine the feasibility of cardiac MRI in patients with pacemakers (PMs) or implantable cardioverter-defibrillators (ICDs).
Thirty-eight patients underwent a total of 39 (8 ICDs and 31 PM) cardiac MRI examinations at 1.5 T using usual protocols without specific absorption rate (SAR) restrictions. Nine PM-dependent patients were included. All devices were interrogated before and immediately after MRI. During the scan, pacing mode was programmed to asynchronous for PM-dependent patients whereas ICDs were programmed to a monitor-only mode. All devices were functioning appropriately after cardiac MRI. Comparison of device parameters obtained before and immediately after MRI revealed no significant changes in pacing threshold, lead impedance, battery status, or sensing signal amplitude. Neither clinical events nor patient complaints were reported. Significant imaging artefacts were present on 11 of 39 scans (28.2%). These artefacts were significantly more frequent in ICDs (8 of 8, 100%) vs. PMs (3 of 31, 9.7%) (P < 0.001). Diagnostic questions were answered in 92.3% of the cases, with just three pronounced artefacts preventing an adequate diagnosis in three ICD patients.
Our results suggest that cardiac MRI may be performed safely in appropriately selected patients with close monitoring during the scan without limitation of peak SAR level using several precautionary measures. Image artefacts were more frequent in ICD patients.
Introduction
Advances in magnetic resonance imaging (MRI) and angiography have led MR to evolve into the diagnostic imaging modality of choice for many musculoskeletal and central nervous system disorders.1,2 Parallel to the growth and evolution of MRI, there is an increasing number of patients benefiting from cardiovascular implantable electronic devices, including pacemakers (PMs), implantable cardioverter-defibrillators (ICDs), cardiac resynchronization therapy (CRT) devices, and implantable loop recorders. As a result, the probability of a patient being indicated for an MRI over the lifetime of his device has been estimated in 50–75%.3
Until recently, the presence of either a PM or an ICD has been considered an absolute contraindication to undergoing MRI because of the potential adverse interactions effects of strong magnetic fields on PM and ICD systems, including possible movement of the device, induction of ventricular fibrillation, programming changes, PM inhibition, and induced lead currents that lead to tissue heating.4–6 Despite these concerns, several individual cases and a limited number of animal and human studies have been reported on the effects and safety of non-cardiac MRI and devices, suggesting that MRI in pacemaker patients may be performed with a reasonable risk–benefit ratio, as long as specific safety precautions are followed.7–20 Three relatively recent position statements agree with this revolutionary statement.21–23
However, there is controversial data regarding the potential risks of performing specific cardiac MRIs in patients with cardiovascular implantable electronic devices. Some reports have suggested that cardiac and thoracic MRI might be riskier owing to greater radiofrequency power deposition over the region containing the device.24–26 For that reason cardiac MRI has been rare 12,20 or even excluded from many published series.19,27 Following the current safety protocols for MRI in device patients and on a case-by-case bases, the aim of our study was to evaluate the safety and suitability of performing cardiac MRI in a consecutive series of PM and ICD patients.
Methods
Study subjects
Eligible subjects consisted of consecutive patients with implanted permanent PM or ICD referred by their cardiologist for medically necessary cardiac MRI from October 2007 to September 2010. Indications for cardiac MRI are shown in Table 1. In our institution, cardiac MRI offers a better diagnostic precision when compared with nuclear imaging techniques and for that reason has become the standard of care for cardiac imaging in our centre. Devices must have been in place for >2 months at the time of the scan. Patients with CRT devices and those who had either epicardial or abandoned pacing leads were excluded.
| Gender | |
| Male | 21 (53.8%) |
| Female | 18 (46.2%) |
| Age (years) | 53.8 ± 24.2 |
| Cardiac MRI indication | |
| Ischaemic cardiomyopathy | 20 (51.3%) |
| Myocardial viability | 19 |
| Myocardial perfusion | 1 |
| Congenital heart disease | 13 (33.3%) |
| Right ventricle after Fallot surgery | 6 |
| Systemic ventricle after Mustard surgery | 3 |
| Systemic ventricle in ccTGA patients | 2 |
| Heterotaxia syndrome with single atria | 2 |
| Evaluation of the right ventricle | 4 (10.3%) |
| Arrhythmogenic right ventricular cardiomyopathy | 2 |
| Severe tricuspid regurgitation | 2 |
| Other | 2 (5.1%) |
| Pulmonary vein stenosis after AF ablation | 1 |
| Cardiac amyloidosis | 1 |
| Pacing mode | |
| Pacemaker | |
| VVI | 14 (35.9%) |
| VDD | 6 (15.4%) |
| DDD | 11 (28.2%) |
| ICD | |
| VVEV | 2 (5.1%) |
| VVED | 2 (5.1%) |
| DDED | 4 (10.3%) |
| Manufacturer | |
| Medtronic® | 12 (30.8%) |
| Vitatron® | 4 (10.3%) |
| Biotronik® | 5 (12.8%) |
| Sorin® | 4 (10.3%) |
| St. Jude Medical® | 6 (15.4%) |
| Boston Scientific® | 8 (20.5%) |
| Gender | |
| Male | 21 (53.8%) |
| Female | 18 (46.2%) |
| Age (years) | 53.8 ± 24.2 |
| Cardiac MRI indication | |
| Ischaemic cardiomyopathy | 20 (51.3%) |
| Myocardial viability | 19 |
| Myocardial perfusion | 1 |
| Congenital heart disease | 13 (33.3%) |
| Right ventricle after Fallot surgery | 6 |
| Systemic ventricle after Mustard surgery | 3 |
| Systemic ventricle in ccTGA patients | 2 |
| Heterotaxia syndrome with single atria | 2 |
| Evaluation of the right ventricle | 4 (10.3%) |
| Arrhythmogenic right ventricular cardiomyopathy | 2 |
| Severe tricuspid regurgitation | 2 |
| Other | 2 (5.1%) |
| Pulmonary vein stenosis after AF ablation | 1 |
| Cardiac amyloidosis | 1 |
| Pacing mode | |
| Pacemaker | |
| VVI | 14 (35.9%) |
| VDD | 6 (15.4%) |
| DDD | 11 (28.2%) |
| ICD | |
| VVEV | 2 (5.1%) |
| VVED | 2 (5.1%) |
| DDED | 4 (10.3%) |
| Manufacturer | |
| Medtronic® | 12 (30.8%) |
| Vitatron® | 4 (10.3%) |
| Biotronik® | 5 (12.8%) |
| Sorin® | 4 (10.3%) |
| St. Jude Medical® | 6 (15.4%) |
| Boston Scientific® | 8 (20.5%) |
MRI, magnetic resonance imaging; ccTGA, congenital corrected transposition of the great arteries; AF, atrial fibrillation; ICD, implantable cardioverter defibrillator.
| Gender | |
| Male | 21 (53.8%) |
| Female | 18 (46.2%) |
| Age (years) | 53.8 ± 24.2 |
| Cardiac MRI indication | |
| Ischaemic cardiomyopathy | 20 (51.3%) |
| Myocardial viability | 19 |
| Myocardial perfusion | 1 |
| Congenital heart disease | 13 (33.3%) |
| Right ventricle after Fallot surgery | 6 |
| Systemic ventricle after Mustard surgery | 3 |
| Systemic ventricle in ccTGA patients | 2 |
| Heterotaxia syndrome with single atria | 2 |
| Evaluation of the right ventricle | 4 (10.3%) |
| Arrhythmogenic right ventricular cardiomyopathy | 2 |
| Severe tricuspid regurgitation | 2 |
| Other | 2 (5.1%) |
| Pulmonary vein stenosis after AF ablation | 1 |
| Cardiac amyloidosis | 1 |
| Pacing mode | |
| Pacemaker | |
| VVI | 14 (35.9%) |
| VDD | 6 (15.4%) |
| DDD | 11 (28.2%) |
| ICD | |
| VVEV | 2 (5.1%) |
| VVED | 2 (5.1%) |
| DDED | 4 (10.3%) |
| Manufacturer | |
| Medtronic® | 12 (30.8%) |
| Vitatron® | 4 (10.3%) |
| Biotronik® | 5 (12.8%) |
| Sorin® | 4 (10.3%) |
| St. Jude Medical® | 6 (15.4%) |
| Boston Scientific® | 8 (20.5%) |
| Gender | |
| Male | 21 (53.8%) |
| Female | 18 (46.2%) |
| Age (years) | 53.8 ± 24.2 |
| Cardiac MRI indication | |
| Ischaemic cardiomyopathy | 20 (51.3%) |
| Myocardial viability | 19 |
| Myocardial perfusion | 1 |
| Congenital heart disease | 13 (33.3%) |
| Right ventricle after Fallot surgery | 6 |
| Systemic ventricle after Mustard surgery | 3 |
| Systemic ventricle in ccTGA patients | 2 |
| Heterotaxia syndrome with single atria | 2 |
| Evaluation of the right ventricle | 4 (10.3%) |
| Arrhythmogenic right ventricular cardiomyopathy | 2 |
| Severe tricuspid regurgitation | 2 |
| Other | 2 (5.1%) |
| Pulmonary vein stenosis after AF ablation | 1 |
| Cardiac amyloidosis | 1 |
| Pacing mode | |
| Pacemaker | |
| VVI | 14 (35.9%) |
| VDD | 6 (15.4%) |
| DDD | 11 (28.2%) |
| ICD | |
| VVEV | 2 (5.1%) |
| VVED | 2 (5.1%) |
| DDED | 4 (10.3%) |
| Manufacturer | |
| Medtronic® | 12 (30.8%) |
| Vitatron® | 4 (10.3%) |
| Biotronik® | 5 (12.8%) |
| Sorin® | 4 (10.3%) |
| St. Jude Medical® | 6 (15.4%) |
| Boston Scientific® | 8 (20.5%) |
MRI, magnetic resonance imaging; ccTGA, congenital corrected transposition of the great arteries; AF, atrial fibrillation; ICD, implantable cardioverter defibrillator.
Study protocol
Prior to the scan, patients were counselled regarding the risks of the cardiac MRI, and gave written informed consent after demonstrating an understanding of potential risks, which included irreversible damage to the device, thermal injury, and device malfunction leading to arrhythmia. All devices were interrogated before and immediately after MRI. Device parameters including pacing mode, battery voltage and impedance, lead capture threshold at a fixed pulse width, lead impedances, and sensing signal amplitudes were recorded at each interrogation. Significant changes in pacing thresholds were defined as a difference of at least 1 V between pre- and post-MRI values.15 Pacemaker dependency was assessed before MRI scanning and was defined as the absence of intrinsic rhythm during transient programming of the device to ventricular-inhibited pacing at a backup rate of 40 ppm. Pacing mode was programmed to asynchronous (V00 or D00) for PM-dependent patients and was unchanged in the rest of patients.28 Implantable cardioverter-defibrillators were programmed to a monitor-only mode (arrhythmia detection on, therapies off). Defibrillation threshold testing post-MRI was not performed.29,30
All cardiac MRI studies were performed using an MR system operating at a static magnetic field strength of 1.5 T (Siemens® Magneton-Avanto® MR-2004-V, Erlangen, Germany.) Heart rate and oxygen saturation were monitored continuously with magnetic resonance compatible optically encoded ECG and pulse oximetry (Invivo® 3155MVS, Orlando, FL, USA). Audio contact was established via an intercom system, and patients were asked to inform the investigator immediately of any torque or heating sensation, pain, dizziness, palpitations, or other symptoms during imaging. An experienced cardiologist and full resuscitation equipment were available in the MRI suite during all examinations. The radiologist who led the scan was asked about the existence of any artefact or technical limitation during the test caused by the PM or ICD. Three categories were characterized based on the importance of the artefact: (i) cases in which a little artefact is present in the area adjacent to the generator (Group A), (ii) cases in which a bigger artefact is reported without limiting MRI diagnosis (Group B), and (iii) cases where the artefact prevents an adequate diagnosis (Group C). We defined as significant artefacts Groups B and C. Specific absorption rate (SAR), called into question as a predictor of local tissue heating, was not limited.14,29 Scans were performed using usual protocols with standard SAR settings for the scan. The study was performed in accordance with the Declaration of Helsinki and approved by the local institutional review board.
Statistics
The data were normally distributed (assessed by Shapiro–Wilk test) and were summarized as mean and SEM. Discrete variables were summarized as absolute numbers and percentages. Pre- and post-MRI parameters (battery voltage and impedance, lead capture threshold, lead impedances, and sensing signal amplitudes) were compared with the paired Student's t-test. Contingency tables were used to analyse possible relationships between discrete variables. If an expected cell count for any 2 × 2 crosstabs is <5, the reported P-value is from Fisher exact test. Otherwise, the reported P-values are from the continuity-corrected chi-square. Analyses were performed with SPSS statistical software (version 13.0). All tests were two-tailed and the level of significance was set to a value of 0.05.
Results
The study group consisted of 38 consecutive patients on which a total of 39 cardiac MRI examinations were performed. Thirty-one pacemakers (9 PM dependent) and 8 ICDs were analysed; this yielded data on 56 leads, of which 39 were ventricular and 17 were atrial leads. The characteristics of the patients and devices are presented in Table 1.
Safety and device function
All devices were functioning appropriately after cardiac MRI. Comparison of device parameters obtained before and immediately after MRI revealed no significant changes in pacing thresholds, leads impedances, battery status, or sensing signal amplitudes (Table 2). Pacing threshold changed slightly in 9 of 56 leads (16.1%), including 5 atrial (29.4%) and 4 ventricular leads (10.2%) (P = 0.084). The maximum pacing threshold change was a 0.8-V decrease in an atrial lead (Figure 1). No single case of significant threshold change was registered considering the pre-specified criteria and no changes in device programming were observed. None of the patients reported any torque or heating sensations, palpitations, dizziness, or other unusual symptoms. No procedures were terminated owing to clinical events or patient complaints.
Comparison of device parameters obtained before and immediately after cardiac magnetic resonance imaging. Results are summarized as mean ± SD
| . | Before cardiac MRI . | After cardiac MRI . | P value . |
|---|---|---|---|
| Ventricular capture threshold (V) | 0.8 ± 0.39 | 0.79 ± 0.41 | 0.379 |
| Auricular capture threshold (V) | 1.01 ± 0.39 | 0.95 ± 0.45 | 0.402 |
| Ventricular sensing (mV) | 10.33 ± 4.19 | 10.36 ± 4.16 | 0.893 |
| Auricular sensing (mV) | 2.81 ± 2.39 | 2.8 ± 2.45 | 0.901 |
| Ventricular lead impedance (Ω) | 529.92 ± 197.64 | 533.33 ± 204.73 | 0.512 |
| Auricular lead impedance (Ω) | 495.28 ± 218.61 | 496.33 ± 216.95 | 0.891 |
| Battery voltage (V) | 2.86 ± 0.13 | 2.86 ± 0.14 | 0.901 |
| Battery impedance (Ω) | 335.26 ± 287.52.99 | 337.76 ± 295.96 | 0.443 |
| . | Before cardiac MRI . | After cardiac MRI . | P value . |
|---|---|---|---|
| Ventricular capture threshold (V) | 0.8 ± 0.39 | 0.79 ± 0.41 | 0.379 |
| Auricular capture threshold (V) | 1.01 ± 0.39 | 0.95 ± 0.45 | 0.402 |
| Ventricular sensing (mV) | 10.33 ± 4.19 | 10.36 ± 4.16 | 0.893 |
| Auricular sensing (mV) | 2.81 ± 2.39 | 2.8 ± 2.45 | 0.901 |
| Ventricular lead impedance (Ω) | 529.92 ± 197.64 | 533.33 ± 204.73 | 0.512 |
| Auricular lead impedance (Ω) | 495.28 ± 218.61 | 496.33 ± 216.95 | 0.891 |
| Battery voltage (V) | 2.86 ± 0.13 | 2.86 ± 0.14 | 0.901 |
| Battery impedance (Ω) | 335.26 ± 287.52.99 | 337.76 ± 295.96 | 0.443 |
Comparison of device parameters obtained before and immediately after cardiac magnetic resonance imaging. Results are summarized as mean ± SD
| . | Before cardiac MRI . | After cardiac MRI . | P value . |
|---|---|---|---|
| Ventricular capture threshold (V) | 0.8 ± 0.39 | 0.79 ± 0.41 | 0.379 |
| Auricular capture threshold (V) | 1.01 ± 0.39 | 0.95 ± 0.45 | 0.402 |
| Ventricular sensing (mV) | 10.33 ± 4.19 | 10.36 ± 4.16 | 0.893 |
| Auricular sensing (mV) | 2.81 ± 2.39 | 2.8 ± 2.45 | 0.901 |
| Ventricular lead impedance (Ω) | 529.92 ± 197.64 | 533.33 ± 204.73 | 0.512 |
| Auricular lead impedance (Ω) | 495.28 ± 218.61 | 496.33 ± 216.95 | 0.891 |
| Battery voltage (V) | 2.86 ± 0.13 | 2.86 ± 0.14 | 0.901 |
| Battery impedance (Ω) | 335.26 ± 287.52.99 | 337.76 ± 295.96 | 0.443 |
| . | Before cardiac MRI . | After cardiac MRI . | P value . |
|---|---|---|---|
| Ventricular capture threshold (V) | 0.8 ± 0.39 | 0.79 ± 0.41 | 0.379 |
| Auricular capture threshold (V) | 1.01 ± 0.39 | 0.95 ± 0.45 | 0.402 |
| Ventricular sensing (mV) | 10.33 ± 4.19 | 10.36 ± 4.16 | 0.893 |
| Auricular sensing (mV) | 2.81 ± 2.39 | 2.8 ± 2.45 | 0.901 |
| Ventricular lead impedance (Ω) | 529.92 ± 197.64 | 533.33 ± 204.73 | 0.512 |
| Auricular lead impedance (Ω) | 495.28 ± 218.61 | 496.33 ± 216.95 | 0.891 |
| Battery voltage (V) | 2.86 ± 0.13 | 2.86 ± 0.14 | 0.901 |
| Battery impedance (Ω) | 335.26 ± 287.52.99 | 337.76 ± 295.96 | 0.443 |
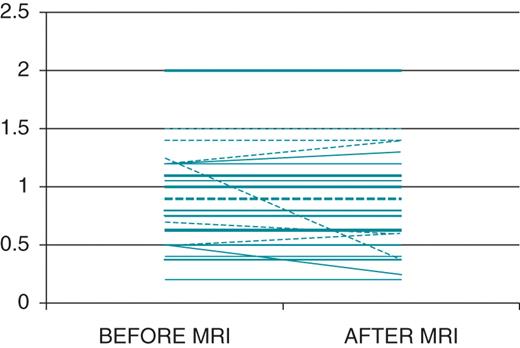
Pacing threshold value modifications after cardiac magnetic resonance imaging. Dashed lines represent atrial leads and continuous lines represent ventricular leads. Thicker lines represent more than one device in the same value.
Specific absorption rate values did not exceed FDA recommendations during any of the studies. The median peak SAR was 2.9 W/kg. Ventricular over-sensing of electromagnetic noise occurred in one (3.8%) ICD (Boston Scientific® Vitality2DR®). The noise was interpreted by the device as ventricular fibrillation (Figure 2). Fortunately, the safety protocol had been followed (the ICD had been programmed to a monitor-only mode) and consequently this electromagnetic interference had no clinical implication.
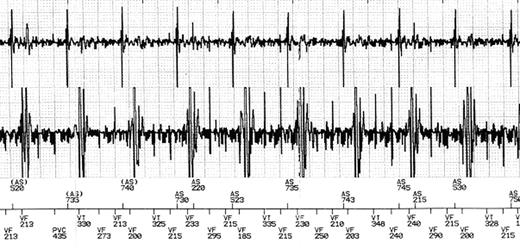
Rhythm strip stored by a Boston Scientific® Vitality 2DR® implantable cardioverter-defibrillator during cardiac magnetic resonance imaging. Endocardial electrogram and event markers (AS, atrial sensing; VT, ventricular tachycardia; VF, ventricular fibrillation). Even although stable sinus rhythm was confirmed by continuous ECG monitoring, a ventricular fibrillation episode was reported due to oversensing of radio frequency fields.
Image quality and diagnostic yield
Little bright and dark artefacts just around the generator (Group A) were present on 28 of 39 scans; all of them are PM patients. Artefacts with spatial distortion of surrounding anatomy without limiting the diagnosis (Group B) were described on 8 of 39 scans (3 PM and 5 ICDs). Three pronounced artefacts were observed in three scans of ICD patients. These artefacts prevented reaching the diagnosis in these cases (Group C). Significant artefacts (Groups B and C) were more frequent in ICDs (9.7% of PM vs. 100% of ICD; P < 0.001) (Figure 3). Image distortion was most pronounced on inversion recovery and cine true fast with steady-state precession sequences and on image planes roughly parallel to the devices. On the other hand, fast gradient recalled echo, tagging and fast-spoiled gradient recalled echo sequences provided good image quality (Figure 4).
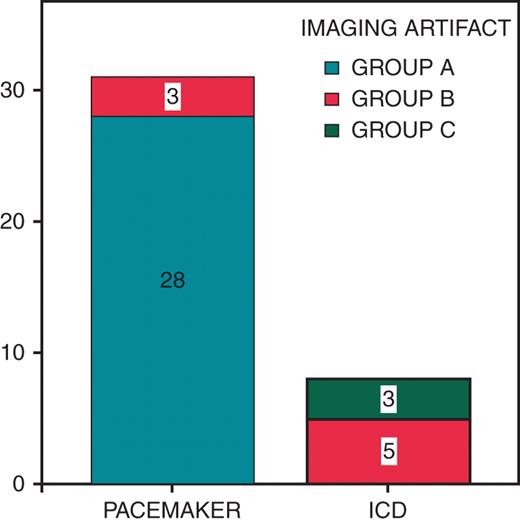
Frequency of image artefacts depending on performing cardiac magnetic resonance imaging in pacemaker or implantable cardioverter-defibrillator patients. Group A: little artefact is present in the area adjacent to the generator. Group B: a bigger artefact is reported without limiting magnetic resonance imaging diagnosis. Group C: cases where the artefact prevents an adequate diagnosis.
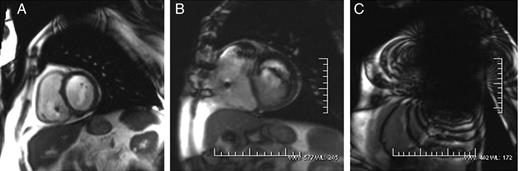
Cine true fast with steady-state precession (trueFISP) sequences during cardiac MRI from three different device patients. Case A shows little image artefact in the area adjacent to the generator (Group A). The imaging artefact in the case B was bigger but did not limit the MR diagnosis (Group B). Case C shows a severe imaging artefact which prevented reaching the diagnosis.
Discussion
Because of its superior spatial and tissue contrast resolution, multiplanar imaging capability, and lack of ionizing radiation, MRI has become an invaluable clinical tool in all fields, including cardiovascular diseases. Patients with complex structural heart diseases frequently have poor echocardiographic windows and will require serial cardiac MRI for both structural assessment and quantification of flow and ventricular function, especially of the right ventricle. Regrettably, these patients have a high likelihood of eventually requiring a pacing system for heart block, sinus node dysfunction, or tachycardia management. The safety of cardiac MRI in these patients is controversial. In the past, the presence of a cardiac device was considered a strict contraindication for a patient in the MR environment. More recently, various studies have reported how to perform safely a non-cardiac MRI in patients with implanted devices in carefully selected clinical circumstances and using appropriate strategies, which have been finally defined in different expert consensus documents.8–24 However, there is limited data regarding specific cardiac MRI.24,25
To our knowledge, the current study is the first series focusing on the safety and feasibility of specific cardiac MRI in patients with implantable cardiac devices. In this series of 39 consecutive cases, the performance of necessary cardiac MRI procedures using a 1.5 T MR system without SAR restriction was found to have an acceptable safety profile as long as a comprehensive safety protocol was used. Patients remained asymptomatic during the scan and no significant change in pacing and sensing parameters was recorded. Considering the entire population sample, significant image artefacts preventing to reach the final diagnosis were present in 7.7% of cases (3 of 39), all of them occurring in ICD patients. Neither clinical heating nor significant change in pacing threshold (>1 V) as an indirect sign of thermal injury of the electrode–tissue boundary was identified. Small changes in pacing threshold were more frequents in atrial leads, which is in concordance with previously reported observations.19
One of the most important findings of this study is the presence of significant image artefacts in the eight ICDs included, preventing the radiologist to reach the diagnosis in three cases. Previous studies have reported how an MR image might be altered by the presence of ferromagnetic materials leading to image artefacts.16,17 Our results highlight that this is more frequent in cardiac MRI of ICD patients due to both the proximity and the size of the device.16,26,30 This could be an important limitation to cardiac MRI in this setting, considering that an adequate diagnosis could not be achieved in up to 37.5% of the ICD patients (three of eight studies). None of the ICD patients underwent defibrillator threshold testing after MRI as currently recommended by the American Heart Association. However, recent published data suggest that routinely post-MRI defibrillator threshold testing in this setting may not be necessary.31 The use of asynchronous pacing in non-pacemaker-dependent patients during the scan has been fiercely criticized,27,32,33 proposing 0V0, 0D0, 000, or subthreshold programming as an alternative. We believed that induction of tachyarrhythmias during asynchronous pacing is extremely rare and our strategy followed the general recommendations in force when the study began.23,34
The SAR value was not limited in the present study. Attempts to limit it may lead to suboptimal scans, adjusting flip angles may adversely affect the signal-to-noise ratio, reducing matrix size may decrease scan resolution and increasing repetition time may prolong scan time. The current scientific statement from the American Heart Association22 does not comment on SAR limitations, although the European Society of Cardiology recommends an SAR limit of 2 W/kg.23 Recently, the practice of using peak SAR calculations to predict temperature changes at the lead tip-tissue interface has come into question14,29 whereas more recent statements plead for limiting this parameter as an additional safety measure.35
Study limitations
This study is limited by its lack of objective measurement of any changes in temperature of the implanted system. However, no single patient reported local heating, and there were no signs of PM or ICD malfunction. The present findings are limited to 1.5 T scanners and to the PM and ICD models tested in the study. As no CRT devices were included, conclusions regarding this subgroup of devices cannot be drawn. A major limitation of the study is the fact that no intermediate or long-term follow up data are provided and thus possible significant changes in device operation occurring after immediate post-MRI evaluation could have been undetected. Only eight ICD patients were enrolled in this study, so definitive conclusions regarding this subgroup should be taken cautiously. Although lead and implant geometry may play a role in lead heating, these variables were not included in the study.36 Given the infinite possibilities of electromagnetic interferences and data published previously, the absolute safety of PM and ICD and MRI interactions cannot be assured. While the results are encouraging, readers should not assume that cardiac MRI may be performed without limitations.
Conclusions
The results of the present study suggest that specific cardiac MRI can be safely performed in selected patients using a comprehensive safety protocol with close monitoring during the scan, and without limitation of peak SAR level. All patients remained asymptomatic during the scan and no significant changes in pacing or sensing parameters were recorded. Significant image artefacts were more frequent in ICD patients. While the safety of this procedure is definitively established, we suggest a selection of device patients on a case-by-case basis when the diagnostic benefit from MRI outweighs the presumed risks.
Conflict of interest: none declared.



