-
PDF
- Split View
-
Views
-
Cite
Cite
Yuki Komatsu, Kikuya Uno, Kiyoshi Otomo, Yasutoshi Nagata, Hiroshi Taniguchi, Kazuyoshi Ogura, Yasuyuki Egami, Kei Takayama, Ken Kakita, Yoshito Iesaka, Atrial defibrillation threshold as a novel predictor of clinical outcome of catheter ablation for persistent atrial fibrillation, EP Europace, Volume 13, Issue 2, February 2011, Pages 213–220, https://doi.org/10.1093/europace/euq357
Close - Share Icon Share
Abstract
Catheter ablation for persistent atrial fibrillation (AF) is currently performed with different procedural endpoints. When AF did not terminate during ablation procedure, electrical cardioversion was performed at different defibrillation threshold (DFT) according to AF characteristics and atrial electrophysiologic substrates. We sought to evaluate the impact of atrial DFT after catheter ablation for persistent AF on clinical outcome.
We studied 128 patients with persistent AF (age 63 ± 9 years, 106 men). After completion of circumferential pulmonary vein isolation, the left atrial substrate ablation was performed until AF terminated or all identified complex fractionated electrograms were eliminated. If AF did not terminate during ablation, an internal cardioversion protocol was started at 5J and was increased incrementally in 5 J steps until successful cardioversion was accomplished. Procedural AF termination was achieved in 50 patients (Group A). Atrial fibrillation was terminated by cardioversion with DFT ≤ 10 J in 47 patients (Group B) and with DFT > 10 J in 31 patients (Group C). At 14 ± 7 follow-up months after 1.3 ± 0.5 sessions, 47 (94%) Group A patients, 42 (89%) Group B patients, and 14 (45%) Group C patients remained in sinus rhythm. In multivariate analysis of Group B and Group C, DFT (hazard ratio 5.54, P< 0.001) and AF duration (hazard ratio 3.74, P= 0.011) were independent predictors of recurrent arrhythmia.
When AF does not terminate after the completion of predetermined stepwise ablation, further extensive ablation to terminate AF might be unnecessary if the AF can be successfully terminated by electrical cardioversion at low DFT.
Introduction
Catheter ablation targeting elimination of the triggers of atrial fibrillation (AF) through electrical isolation of the pulmonary veins (PV) has been the basis of most AF ablation approaches to date.1–4 Whereas catheter ablation with the endpoint of PV isolation has yielded encouraging success rates in paroxysmal AF, such success has not been achieved in patients with persistent AF.5–7 Successful elimination of persistent AF has become possible with the introduction of linear or electrogram-based ablation techniques.8–15 Although previous studies found a correlation between procedural AF termination and better clinical outcome,11–15 more extensive ablation is required to terminate persistent AF and may result in a higher possibility of complications. When termination of AF cannot be achieved during ablation, internal electrical cardioversion is a reliable procedure to restore sinus rhythm. Previous studies have shown that atrial defibrillation threshold (DFT) differs according to AF characteristics and atrial electrophysiologic substrates.16–19 Atrial substrates with high global dominant fibrillatory frequency and low AF organization require higher energy levels to attain successful defibrillation. However, there is no consensus on the relation between DFT after ablation and clinical outcome of persistent AF. The purpose of this study was to evaluate the impact of DFT after ablation on the clinical outcome of patients with persistent AF.
Methods
Study subjects
The subjects of the present study were 128 patients undergoing catheter ablation for persistent AF. There were 106 men and 22 women, and their mean age was 63 ± 9 years (range 29–79 years). Mean left atrial (LA) size was 46.3 ± 5.4 mm. Structural heart disease was present in 32 patients (25%), included hypertensive heart disease in 8 (6%), dilated cardiomyopathy in 5 (4%), coronary artery disease in 14 (11%), and valvular heart disease in 5 (4%). According to HRS/EHRA/ECAS 2007 Consensus Statement on Catheter Surgical Ablation of AF,20 persistent AF was defined as AF that is sustained beyond 7 days or that lasts <7 days but necessitates cardioversion, and long-standing persistent AF was defined as AF that had been present for more than 1 year without intervening spontaneous episodes of sinus rhythm. The mean duration of sustained AF was 57 ± 57 months (median 48 months, range 3–300 months), and 97 (76%) patients were defined as having long-standing persistent AF.
Ablation procedure
All patients gave their written informed consent before the ablation procedures. All antiarrhythmic drugs except amiodarone were discontinued for at least five half-lives prior to the procedure. In the 30 patients previously receiving amiodarone, the drug was stopped at least 1 month prior to the procedure. Patients were on oral anticoagulation to maintain a target international normalized ratio of 1.8–2.5 for at least 1 month before the procedure, and anticoagulation was maintained with intravenous heparin following warfarin discontinuation 3 days prior to the procedure. Surface electrocardiogram (ECG) and bipolar endocardial electrograms were continuously monitored and stored on a computer-based digital amplifier (Labsystem Pro, Bard Electrophysiology, MA, USA). Following transseptal puncture, an intravenous bolus of 100IU/kg body weight of heparin was administered, and heparinized saline was additionally infused to maintain the activated clotting time at 300–350 s.
The stepwise approach included circumferential PV isolation and atrial substrate ablation. Intra-PV electrograms were monitored with double decapolar circumferential mapping catheters (Lasso, Biosense-Webster, Diamond Bar, CA, USA) positioned in both the ipsilateral superior and inferior PVs. Encircling continuous radiofrequency lesions surrounding both ipsilateral PV antrums were created until the local electrogram inside the encircled area disappeared or was dissociated. Complete PV isolation was confirmed by the inability to conduct to the LA after pacing at several sites within the PV antrum. Radiofrequency energy of 35–45W was delivered with 4 mm tip non-irrigated catheter (NaviStar, Biosense-Webster) at a target temperature of 50°C for 25–35 s.
If AF did not terminate after completion of PV isolation, atrial substrate ablation was performed sequentially on the LA roof, the inferior aspect of the LA posterior wall, the LA septum, along the mitral annulus, the base of the LA appendage, and on the LA anterior wall, targeting any of the following electrogram features: fractionated or displayed continuous electrical activity, or repetitive rapid activity with a cycle length ≤120 ms or shorter than the cycle length in the LA appendage. Radiofrequency energy of 35–45 W was applied by 8 mm tip conventional catheter in a temperature-controlled fashion at a temperature setting of 50–55°C and duration of 25–35 s. The endpoint of ablation was termination of AF or transformation of all identified complex fractionated atrial electrograms into discrete electrograms and slowing of local cycle length compared with the LA appendage cycle length. Termination of AF was defined as conversion directly to sinus rhythm or to an atrial tachycardia, which was defined as an organized atrial rhythm with a stable cycle length and consistent endocardial activation sequence in both atria. Atrial tachycardia was subsequently mapped with entrainment manoeuvres and ablated without routine use of three-dimensional electroanatomical tools. If AF terminated to sinus rhythm, AF induction was not performed. When sinus rhythm was not restored during ablation, patients underwent internal electrical cardioversion.
Cavotricuspid isthmus ablation was performed after the completion of circumferential PV isolation and atrial substrate ablation in all patients with the endpoint of bidirectional conduction block.
Internal electrical cardioversion protocol
Internal electrical cardioversion was performed from the 6F 7 + 7-polar catheter (Inquiry, St. Jude Medical, St. Paul, MN, USA) placed into the distal coronary sinus (CS) and along the lateral wall of the right atrium (RA), with each group of seven electrodes having a total surface area of 1.8 cm2, 3 mm interelectrode spacing, and electrode length of 4.6 cm (Figure 1). A 5F quadripolar catheter was positioned in the right ventricular apex to provide accurate R-wave synchronization. Three Lasso catheters were positioned in the left superior PV, the left inferior PV, and the right superior PV to confirm any ectopic activity from the PVs initiating immediate recurrence of AF after cardioversion, if it existed. The defibrillation catheter was connected to an external defibrillator (RA positive, CS negative). After the patient was adequately anesthetized with thiopental sodium, a biphasic direct current shock synchronized to the R-wave was delivered from the CS electrodes to the RA electrodes. Biphasic truncated shocks were delivered with two phases of 8 ms duration each. The protocol started at 5J and was increased incrementally in 5 J steps with a 1 min interval between shocks until successful cardioversion was accomplished or a maximal energy of 35J was reached. We considered a shock successful if stable sinus rhythm was restored within 1s of a shock. Defibrillation threshol was defined as the lowest direct current electrical energy that converted AF into sinus rhythm.
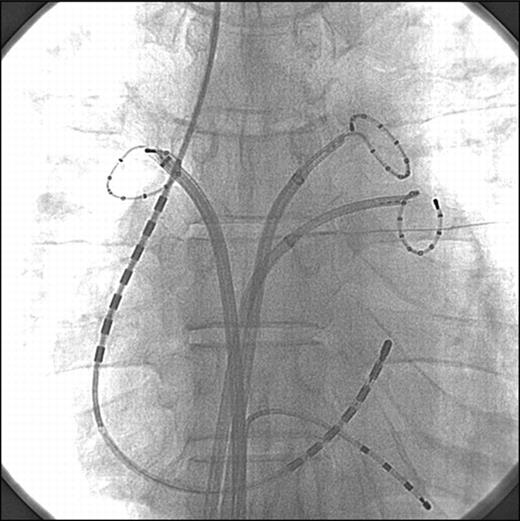
Catheter placement on fluoroscopy during internal electrical cardioversion. Anteroposterior view shows a quadripolar catheter at the right ventricular apex, a 7 + 7-polar catheter in the distal coronary sinus and along the lateral wall of the right atrium, and decapolar ring catheters in the left superior pulmonary vein, left inferior pulmonary vein, and right superior pulmonary vein.
Follow-up
All patients were hospitalized for at least 5 days of ambulatory monitoring following the ablation procedure. The first outpatient clinic visit was 3 weeks after the ablation procedure. The patients then underwent follow-up consisting of clinical interview, ECG, and 24 h Holter monitoring every 1–2 months at our cardiology clinic. If any symptoms suggestive of an arrhythmia occurred between scheduled visits, the patients were asked to come to the emergency department, and ECG, 24 h Holter monitoring, and/or cardiac event recording with a recording duration of 1 month were performed to define the cause of the symptoms. Anticoagulation treatment was discontinued in patients who were confirmed to be in sinus rhythm beyond 6 months after the ablation procedure, if the patient had no history of stroke or was at low risk of thrombo-embolic events. Further continuation of anticoagulation treatment was individualized based on arrhythmia recurrence and patient's risk profile (CHADS2 score). All antiarrhythmic drugs were discontinued within 2 months after ablation. Recurrence of atrial arrhythmias was defined as an episode lasting more than 3 min and that was confirmed by ECGs beyond a blanking period of 2 months after the ablation. A repeat ablation procedure was offered to all patients with recurrent atrial arrhythmias whether symptomatic or not.
Statistical analysis
Continuous data are expressed as mean ± standard deviation and were compared by Student's t-test or by one-way analysis of variance. The Tukey test was performed for post hoc analyses. Categorical data were compared using χ2 analysis or with the Fisher exact test, as appropriate. Arrhythmia-free survival curves were presented as Kaplan–Meier plots and compared by log-rank test. Cox method was used to determine the predictors of recurrent atrial arrhythmia in the patients without procedural AF termination. The following potential predictors of recurrence were considered: age, sex, AF duration, hypertension, hyperlipidaemia, diabetes mellitus, structural heart disease, body mass index, LA diameter, left ventricular ejection fraction, AF cycle length before and after ablation, procedure and radiofrequency application times, and DFT after ablation. Variables whose univariate analyses had a P-value of <0.2 were included in the multivariate Cox regression model. The predictive value of DFT for recurrent arrhythmia after the ablation procedure was assessed using receiver-operator characteristic (ROC) curve. All tests were two-tailed and a P-value of <0.05 was considered statistically significant. Database management and statistical analysis were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA) and graphs using GraphPad Prism version 5.02 (GraphPad Software, San Diego, CA, USA).
Results
Patients and procedure characteristics
Among the 128 patients, procedural AF termination occurred in 50 (39%), of whom AF converted to sinus rhythm directly in 7 patients or via atrial tachycardias in 26 patients. Atrial fibrillation organized into atrial tachycardias that were not successfully mapped and treated by cardioversion in 17 patients. Procedural AF termination did not occur in the remaining 78 (61%) patients, who then underwent internal electrical cardioversion to restore sinus rhythm. The patients without procedural AF termination were further separated according to the median of the DFT into those with DFT of ≤10J (Group B, n= 47) or >10J (Group C, n= 31).
Clinical characteristics and procedural details are shown in Table 1. Clinical variables of patients among the groups were similar except for AF duration and LA diameter. Compared with patients in Group B and Group C, patients in Group A had a significantly shorter AF duration and smaller LA size. There were no significant differences between Group B and Group C in baseline clinical characteristics. Post-ablation AF cycle length was significantly greater in Group A and Group B than in Group C. There was no statistical difference in post-ablation AF cycle length between Group A and Group B.
| . | AF termination (n= 50) . | Non-AF termination (n= 78) . | P-value . | |
|---|---|---|---|---|
| . | . | DFT ≤ 10J (n= 47) . | DFT > 10J (n= 31) . | . |
| Age, years | 62 ± 9 | 62 ± 10 | 65 ± 8 | 0.24 |
| Male | 40 (80) | 40 (85) | 26 (84) | 0.79 |
| Body mass index, kg/m2 | 24.0 ± 3.2 | 24.6 ± 3.1 | 24.8 ± 2.9 | 0.48 |
| AF duration, months | 36.7 ± 39.2 | 60.7 ± 63.8* | 84.0 ± 59.5* | <0.001 |
| Long-standing persistent AF | 33 (66) | 38 (81) | 26 (84) | 0.11 |
| Failed antiarrhythmic drugs | 1.6 ± 0.6 | 1.8 ± 1.2 | 1.8 ± 1.2 | 0.42 |
| Amiodarone use | 13 (26) | 10 (21) | 7 (23) | 0.85 |
| Hypertension | 20 (40) | 16 (34) | 10 (32) | 0.74 |
| Hyperlipidaemia | 14 (28) | 12 (26) | 12 (39) | 0.43 |
| Diabetes mellitus | 5 (10) | 4 (9) | 1 (3) | 0.53 |
| Structural heart disease | 14 (28) | 11 (23) | 7 (23) | 0.82 |
| LA diameter, mm | 44.7 ± 5.3 | 47.1 ± 5.9* | 47.7 ± 4.4* | 0.025 |
| LVEF, % | 61.3 ± 9.4 | 62.4 ± 5.7 | 63.7 ± 7.1 | 0.39 |
| Pre-ablation AFCL, ms | 152.0 ± 21.4 | 146.9 ± 19.6 | 145.3 ± 19.3 | 0.29 |
| Post-ablation AFCL, ms | 182.9 ± 14.9** | 177.3 ± 16.8** | 160.1 ± 15.7 | <0.001 |
| Radiofrequency time, min | ||||
| Total | 74.6 ± 13.1 | 78.8 ± 15.3 | 81.2 ± 11.3 | 0.16 |
| Atrial substrate ablation | 29.9 ± 7.5 | 33.6 ± 7.8 | 33.0 ± 6.7 | 0.09 |
| Procedural time, min | 166.2 ± 23.0 | 174.8 ± 23.9 | 177.8 ± 19.3 | 0.11 |
| Fluoroscopic time, min | 43.7 ± 10.2 | 47.4 ± 10.2 | 44.4 ± 9.8 | 0.28 |
| . | AF termination (n= 50) . | Non-AF termination (n= 78) . | P-value . | |
|---|---|---|---|---|
| . | . | DFT ≤ 10J (n= 47) . | DFT > 10J (n= 31) . | . |
| Age, years | 62 ± 9 | 62 ± 10 | 65 ± 8 | 0.24 |
| Male | 40 (80) | 40 (85) | 26 (84) | 0.79 |
| Body mass index, kg/m2 | 24.0 ± 3.2 | 24.6 ± 3.1 | 24.8 ± 2.9 | 0.48 |
| AF duration, months | 36.7 ± 39.2 | 60.7 ± 63.8* | 84.0 ± 59.5* | <0.001 |
| Long-standing persistent AF | 33 (66) | 38 (81) | 26 (84) | 0.11 |
| Failed antiarrhythmic drugs | 1.6 ± 0.6 | 1.8 ± 1.2 | 1.8 ± 1.2 | 0.42 |
| Amiodarone use | 13 (26) | 10 (21) | 7 (23) | 0.85 |
| Hypertension | 20 (40) | 16 (34) | 10 (32) | 0.74 |
| Hyperlipidaemia | 14 (28) | 12 (26) | 12 (39) | 0.43 |
| Diabetes mellitus | 5 (10) | 4 (9) | 1 (3) | 0.53 |
| Structural heart disease | 14 (28) | 11 (23) | 7 (23) | 0.82 |
| LA diameter, mm | 44.7 ± 5.3 | 47.1 ± 5.9* | 47.7 ± 4.4* | 0.025 |
| LVEF, % | 61.3 ± 9.4 | 62.4 ± 5.7 | 63.7 ± 7.1 | 0.39 |
| Pre-ablation AFCL, ms | 152.0 ± 21.4 | 146.9 ± 19.6 | 145.3 ± 19.3 | 0.29 |
| Post-ablation AFCL, ms | 182.9 ± 14.9** | 177.3 ± 16.8** | 160.1 ± 15.7 | <0.001 |
| Radiofrequency time, min | ||||
| Total | 74.6 ± 13.1 | 78.8 ± 15.3 | 81.2 ± 11.3 | 0.16 |
| Atrial substrate ablation | 29.9 ± 7.5 | 33.6 ± 7.8 | 33.0 ± 6.7 | 0.09 |
| Procedural time, min | 166.2 ± 23.0 | 174.8 ± 23.9 | 177.8 ± 19.3 | 0.11 |
| Fluoroscopic time, min | 43.7 ± 10.2 | 47.4 ± 10.2 | 44.4 ± 9.8 | 0.28 |
Data are presented as mean ± SD or n (%). Hyperlipidaemia is defined as cholesterol of at least 220 mg/dL. AF, atrial fibrillation; AFCL, atrial fibrillation cycle length; DFT, defibrillation threshold; LA, left atrial; LVEF, left ventricular ejection fraction.
*P < 0.05 (Turkey test) when compared with patients of AF termination.
**P < 0.05 (Turkey test) when compared with patients of DFT > 10 J.
| . | AF termination (n= 50) . | Non-AF termination (n= 78) . | P-value . | |
|---|---|---|---|---|
| . | . | DFT ≤ 10J (n= 47) . | DFT > 10J (n= 31) . | . |
| Age, years | 62 ± 9 | 62 ± 10 | 65 ± 8 | 0.24 |
| Male | 40 (80) | 40 (85) | 26 (84) | 0.79 |
| Body mass index, kg/m2 | 24.0 ± 3.2 | 24.6 ± 3.1 | 24.8 ± 2.9 | 0.48 |
| AF duration, months | 36.7 ± 39.2 | 60.7 ± 63.8* | 84.0 ± 59.5* | <0.001 |
| Long-standing persistent AF | 33 (66) | 38 (81) | 26 (84) | 0.11 |
| Failed antiarrhythmic drugs | 1.6 ± 0.6 | 1.8 ± 1.2 | 1.8 ± 1.2 | 0.42 |
| Amiodarone use | 13 (26) | 10 (21) | 7 (23) | 0.85 |
| Hypertension | 20 (40) | 16 (34) | 10 (32) | 0.74 |
| Hyperlipidaemia | 14 (28) | 12 (26) | 12 (39) | 0.43 |
| Diabetes mellitus | 5 (10) | 4 (9) | 1 (3) | 0.53 |
| Structural heart disease | 14 (28) | 11 (23) | 7 (23) | 0.82 |
| LA diameter, mm | 44.7 ± 5.3 | 47.1 ± 5.9* | 47.7 ± 4.4* | 0.025 |
| LVEF, % | 61.3 ± 9.4 | 62.4 ± 5.7 | 63.7 ± 7.1 | 0.39 |
| Pre-ablation AFCL, ms | 152.0 ± 21.4 | 146.9 ± 19.6 | 145.3 ± 19.3 | 0.29 |
| Post-ablation AFCL, ms | 182.9 ± 14.9** | 177.3 ± 16.8** | 160.1 ± 15.7 | <0.001 |
| Radiofrequency time, min | ||||
| Total | 74.6 ± 13.1 | 78.8 ± 15.3 | 81.2 ± 11.3 | 0.16 |
| Atrial substrate ablation | 29.9 ± 7.5 | 33.6 ± 7.8 | 33.0 ± 6.7 | 0.09 |
| Procedural time, min | 166.2 ± 23.0 | 174.8 ± 23.9 | 177.8 ± 19.3 | 0.11 |
| Fluoroscopic time, min | 43.7 ± 10.2 | 47.4 ± 10.2 | 44.4 ± 9.8 | 0.28 |
| . | AF termination (n= 50) . | Non-AF termination (n= 78) . | P-value . | |
|---|---|---|---|---|
| . | . | DFT ≤ 10J (n= 47) . | DFT > 10J (n= 31) . | . |
| Age, years | 62 ± 9 | 62 ± 10 | 65 ± 8 | 0.24 |
| Male | 40 (80) | 40 (85) | 26 (84) | 0.79 |
| Body mass index, kg/m2 | 24.0 ± 3.2 | 24.6 ± 3.1 | 24.8 ± 2.9 | 0.48 |
| AF duration, months | 36.7 ± 39.2 | 60.7 ± 63.8* | 84.0 ± 59.5* | <0.001 |
| Long-standing persistent AF | 33 (66) | 38 (81) | 26 (84) | 0.11 |
| Failed antiarrhythmic drugs | 1.6 ± 0.6 | 1.8 ± 1.2 | 1.8 ± 1.2 | 0.42 |
| Amiodarone use | 13 (26) | 10 (21) | 7 (23) | 0.85 |
| Hypertension | 20 (40) | 16 (34) | 10 (32) | 0.74 |
| Hyperlipidaemia | 14 (28) | 12 (26) | 12 (39) | 0.43 |
| Diabetes mellitus | 5 (10) | 4 (9) | 1 (3) | 0.53 |
| Structural heart disease | 14 (28) | 11 (23) | 7 (23) | 0.82 |
| LA diameter, mm | 44.7 ± 5.3 | 47.1 ± 5.9* | 47.7 ± 4.4* | 0.025 |
| LVEF, % | 61.3 ± 9.4 | 62.4 ± 5.7 | 63.7 ± 7.1 | 0.39 |
| Pre-ablation AFCL, ms | 152.0 ± 21.4 | 146.9 ± 19.6 | 145.3 ± 19.3 | 0.29 |
| Post-ablation AFCL, ms | 182.9 ± 14.9** | 177.3 ± 16.8** | 160.1 ± 15.7 | <0.001 |
| Radiofrequency time, min | ||||
| Total | 74.6 ± 13.1 | 78.8 ± 15.3 | 81.2 ± 11.3 | 0.16 |
| Atrial substrate ablation | 29.9 ± 7.5 | 33.6 ± 7.8 | 33.0 ± 6.7 | 0.09 |
| Procedural time, min | 166.2 ± 23.0 | 174.8 ± 23.9 | 177.8 ± 19.3 | 0.11 |
| Fluoroscopic time, min | 43.7 ± 10.2 | 47.4 ± 10.2 | 44.4 ± 9.8 | 0.28 |
Data are presented as mean ± SD or n (%). Hyperlipidaemia is defined as cholesterol of at least 220 mg/dL. AF, atrial fibrillation; AFCL, atrial fibrillation cycle length; DFT, defibrillation threshold; LA, left atrial; LVEF, left ventricular ejection fraction.
*P < 0.05 (Turkey test) when compared with patients of AF termination.
**P < 0.05 (Turkey test) when compared with patients of DFT > 10 J.
No statistically significant differences were observed in mean procedure times, mean duration of radiofrequency energy applications, and mean fluoroscopy times among the groups. The complex electrograms were targeted in the LA in all patients. There were no differences among the groups in the incidence of procedural complications. Pericardial tamponade occurred in one Group A patient and one Group B patient and was managed successfully by pericardiocentesis. One patient in each group developed pericarditis that did not require intervention. Only one Group B patient experienced transient cerebrovascular ischaemia, which was resolved within hours after the procedure. No oesophageal fistulae occurred. No significant PV stenosis developed during the procedure or follow-up period.
Clinical outcome after a single ablation procedure
At a mean follow-up of 11 ± 8 months after a single ablation procedure, 75/128 (58%) patients were free from recurrent atrial arrhythmia. Among Group A patients, 4 (8%) had recurrent AF and 10 (20%) had recurrent atrial tachycardia. Among Group B patients, 6 (13%) had recurrent AF and 13 (28%) had recurrent atrial tachycardia. Among Group C patients, 14 (45%) had recurrent AF and 6 (19%) had recurrent atrial tachycardia. Maintenance of sinus rhythm after a single ablation procedure was observed more frequently in Group A and Group B patients than in Group C patients (Group A vs. Group C, P= 0.005; Group B vs. Group C, P= 0.031; Group A vs. Group B, P= 0.53; Figure 2A). The incidence of AF recurrence was significantly higher in Group C patients than in Group A and Group B patients (P< 0.001 and P< 0.001, respectively, Figure 3).

Kaplan–Meier curve analysis of the incidence of recurrent arrhythmia after a single ablation procedure (A) and after the repeat procedure (B). These curves are presented according to whether procedural termination was achieved (Termination) or sinus rhythm was restored by internal electrical cardioversion with a defibrillation threshold of ≤10J (DFT ≤ 10 J) or >10J (DFT > 10J). DFT, defibrillation threshold.
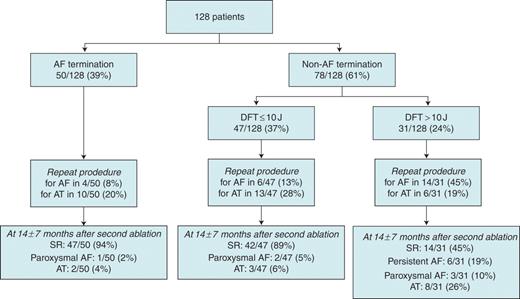
Patient flow diagram. AF, atrial fibrillation; SR, sinus rhythm; AT, atrial tachycardia; DFT, defibrillation threshold.
Repeat ablation procedures
At a mean of 5 ± 3 months after the first procedure, all of the patients with recurrent atrial arrhythmia underwent a second ablation procedure. Repeat ablation procedures were performed for recurrent atrial tachycardia in 29 patients (10 in Group A, 13 in Group B, and 6 in Group C) and for recurrent AF in 24 patients (4 in Group A, 6 in Group B, and 14 in Group C). Electrical reconnection in a mean of 2.0 ± 1.3 PVs per patient was found in 45 of the 53 (85%) patients with recurrent atrial arrhythmia, whereas all PVs remained isolated in eight patients. During the repeat procedures, the recurrent atrial tachycardias were successfully mapped and ablated in 23 of 29 patients (9/10 in Group A, 11/13 in Group B, and 3/6 in Group C). The mechanism of these 23 atrial tachycardias was perimitral (n= 14), cavotricuspid isthmus-dependent (n= 1), and focal (n= 8). Linear ablation of the mitral isthmus was performed for the perimitral tachycardias. Bidirectional conduction block of the mitral isthmus was achieved in 8 of 14 patients. Patients with recurrent AF underwent repeat stepwise ablation with the same strategy as in the initial procedure, and procedural AF termination occurred in 14 of 24 patients (4/4 in Group A, 4/6 in Group B, and 6/14 in Group C). In the remaining 10 of 24 patients, DFT at the repeat procedure was significantly more reduced than that at the first procedure (from 18.5 ± 6.3 to 12.0 ± 5.9J, P= 0.006).
Clinical outcome after the repeat procedure
At a mean follow-up of 14 ± 7 months after the repeat procedure (mean of 1.3 ± 0.5 procedures), 103 of 128 (80%) patients were free from recurrent atrial arrhythmia without antiarrhythmic drug therapy. Maintenance of sinus rhythm was observed in 47 of 50 (94%) patients in Group A, 42 of 47 (89%) patients in Group B (Group A vs. Group B, P= 0.44; Figure 2B), and 14 of 31 (45%) patients in Group C (Group A vs. Group C, P < 0.001; Group B vs. Group C, P < 0.001; Figure 2B). Among Group A patients, 1 (2%) had recurrent paroxysmal AF and 2 (4%) had atrial tachycardia. Among Group B patients, 2 (5%) had paroxysmal AF and 3 (6%) had atrial tachycardia. Among Group C patients, 6 (19%) had recurrent persistent AF, 3 (10%) had paroxysmal AF, and 8 (26%) had atrial tachycardia.
Different defibrillation threshold and clinical outcome
Among Group B and Group C patients, DFT after the ablation was significantly higher in patients with recurrent arrhythmia than in those who maintained sinus rhythm without antiarrhythmic drugs (19.3 ± 7.4 vs. 11.9 ± 5.0J, P < 0.001; Table 2). The ROC curve for DFT after ablation as a predictor of recurrent arrhythmia showed an area under the curve of 0.80 [95% confidence interval
Comparison of characteristics between patients with and without arrhythmia recurrence
| Variable . | Recurrence (n=25) . | No recurrence (n=103) . | P-value . |
|---|---|---|---|
| Age | 65 ± 7.0 | 62 ± 9.6 | 0.14 |
| Male | 20 (80) | 86 (83) | 0.77 |
| Failed antiarrhythmic drugs | 1.9 ± 1.3 | 1.7 ± 0.9 | 0.29 |
| Amiodarone use | 8 (32) | 22 (21) | 0.30 |
| Hypertension | 9 (36) | 37 (36) | 1.00 |
| Hyperlipidaemia | 8 (32) | 30 (29) | 0.81 |
| Diabetes mellitus | 2 (8) | 8 (8) | 1.00 |
| Structure heart disease | 8 (32) | 24 (23) | 0.44 |
| Body mass index, kg/m2 | 24.1 ± 2.8 | 24.5 ± 3.2 | 0.48 |
| AF duration, months | 96.8 ± 55.7 | 47.3 ± 53.2 | <0.001 |
| LA diameter, mm | 48.0 ± 5.6 | 45.9 ± 5.4 | 0.09 |
| LVEF, % | 63.2 ± 7.0 | 61.5 ± 8.7 | 0.30 |
| Pre-ablation AFCL, ms | 146.2 ± 18.5 | 149.0 ± 20.8 | 0.51 |
| Post-ablation AFCL, ms | 171.6 ± 17.1 | 176.2 ± 18.4 | 0.24 |
| DFT after ablation, J | 19.3 ± 7.4 | 11.9 ± 5.0 | <0.001 |
| Procedural time, min | 177.2 ± 26.6 | 171.4 ± 21.9 | 0.41 |
| Radiofrequency time, min | 78.4 ± 15.0 | 77.7 ± 13.4 | 0.88 |
| Variable . | Recurrence (n=25) . | No recurrence (n=103) . | P-value . |
|---|---|---|---|
| Age | 65 ± 7.0 | 62 ± 9.6 | 0.14 |
| Male | 20 (80) | 86 (83) | 0.77 |
| Failed antiarrhythmic drugs | 1.9 ± 1.3 | 1.7 ± 0.9 | 0.29 |
| Amiodarone use | 8 (32) | 22 (21) | 0.30 |
| Hypertension | 9 (36) | 37 (36) | 1.00 |
| Hyperlipidaemia | 8 (32) | 30 (29) | 0.81 |
| Diabetes mellitus | 2 (8) | 8 (8) | 1.00 |
| Structure heart disease | 8 (32) | 24 (23) | 0.44 |
| Body mass index, kg/m2 | 24.1 ± 2.8 | 24.5 ± 3.2 | 0.48 |
| AF duration, months | 96.8 ± 55.7 | 47.3 ± 53.2 | <0.001 |
| LA diameter, mm | 48.0 ± 5.6 | 45.9 ± 5.4 | 0.09 |
| LVEF, % | 63.2 ± 7.0 | 61.5 ± 8.7 | 0.30 |
| Pre-ablation AFCL, ms | 146.2 ± 18.5 | 149.0 ± 20.8 | 0.51 |
| Post-ablation AFCL, ms | 171.6 ± 17.1 | 176.2 ± 18.4 | 0.24 |
| DFT after ablation, J | 19.3 ± 7.4 | 11.9 ± 5.0 | <0.001 |
| Procedural time, min | 177.2 ± 26.6 | 171.4 ± 21.9 | 0.41 |
| Radiofrequency time, min | 78.4 ± 15.0 | 77.7 ± 13.4 | 0.88 |
Data are presented as mean ± SD or n (%). AF, atrial fibrillation; AFCL, atrial fibrillation cycle length; LA, left atrial; LVEF, left ventricle ejection fraction; DFT, defibrillation threshold.
Comparison of characteristics between patients with and without arrhythmia recurrence
| Variable . | Recurrence (n=25) . | No recurrence (n=103) . | P-value . |
|---|---|---|---|
| Age | 65 ± 7.0 | 62 ± 9.6 | 0.14 |
| Male | 20 (80) | 86 (83) | 0.77 |
| Failed antiarrhythmic drugs | 1.9 ± 1.3 | 1.7 ± 0.9 | 0.29 |
| Amiodarone use | 8 (32) | 22 (21) | 0.30 |
| Hypertension | 9 (36) | 37 (36) | 1.00 |
| Hyperlipidaemia | 8 (32) | 30 (29) | 0.81 |
| Diabetes mellitus | 2 (8) | 8 (8) | 1.00 |
| Structure heart disease | 8 (32) | 24 (23) | 0.44 |
| Body mass index, kg/m2 | 24.1 ± 2.8 | 24.5 ± 3.2 | 0.48 |
| AF duration, months | 96.8 ± 55.7 | 47.3 ± 53.2 | <0.001 |
| LA diameter, mm | 48.0 ± 5.6 | 45.9 ± 5.4 | 0.09 |
| LVEF, % | 63.2 ± 7.0 | 61.5 ± 8.7 | 0.30 |
| Pre-ablation AFCL, ms | 146.2 ± 18.5 | 149.0 ± 20.8 | 0.51 |
| Post-ablation AFCL, ms | 171.6 ± 17.1 | 176.2 ± 18.4 | 0.24 |
| DFT after ablation, J | 19.3 ± 7.4 | 11.9 ± 5.0 | <0.001 |
| Procedural time, min | 177.2 ± 26.6 | 171.4 ± 21.9 | 0.41 |
| Radiofrequency time, min | 78.4 ± 15.0 | 77.7 ± 13.4 | 0.88 |
| Variable . | Recurrence (n=25) . | No recurrence (n=103) . | P-value . |
|---|---|---|---|
| Age | 65 ± 7.0 | 62 ± 9.6 | 0.14 |
| Male | 20 (80) | 86 (83) | 0.77 |
| Failed antiarrhythmic drugs | 1.9 ± 1.3 | 1.7 ± 0.9 | 0.29 |
| Amiodarone use | 8 (32) | 22 (21) | 0.30 |
| Hypertension | 9 (36) | 37 (36) | 1.00 |
| Hyperlipidaemia | 8 (32) | 30 (29) | 0.81 |
| Diabetes mellitus | 2 (8) | 8 (8) | 1.00 |
| Structure heart disease | 8 (32) | 24 (23) | 0.44 |
| Body mass index, kg/m2 | 24.1 ± 2.8 | 24.5 ± 3.2 | 0.48 |
| AF duration, months | 96.8 ± 55.7 | 47.3 ± 53.2 | <0.001 |
| LA diameter, mm | 48.0 ± 5.6 | 45.9 ± 5.4 | 0.09 |
| LVEF, % | 63.2 ± 7.0 | 61.5 ± 8.7 | 0.30 |
| Pre-ablation AFCL, ms | 146.2 ± 18.5 | 149.0 ± 20.8 | 0.51 |
| Post-ablation AFCL, ms | 171.6 ± 17.1 | 176.2 ± 18.4 | 0.24 |
| DFT after ablation, J | 19.3 ± 7.4 | 11.9 ± 5.0 | <0.001 |
| Procedural time, min | 177.2 ± 26.6 | 171.4 ± 21.9 | 0.41 |
| Radiofrequency time, min | 78.4 ± 15.0 | 77.7 ± 13.4 | 0.88 |
Data are presented as mean ± SD or n (%). AF, atrial fibrillation; AFCL, atrial fibrillation cycle length; LA, left atrial; LVEF, left ventricle ejection fraction; DFT, defibrillation threshold.
(CI): 0.70–0.89, P < 0.001]. A cut-off value for DFT of 10J after ablation showed a sensitivity of 77.3% and specificity of 75.0% for predicting recurrent arrhythmia. Cox regression revealed the independent predictors of recurrent arrhythmia to be AF duration [hazard ratio, 3.74 (95% CI: 1.36–10.28), P= 0.011] and DFT after ablation [hazard ratio, 5.54 (95% CI: 2.02–15.19), P < 0.001] (Table 3).
Multivariate analyses of the incidence of recurrent arrhythmia in patients without procedural atrial fibrillation termination
| Variable . | Hazard ratio . | 95% Confidence interval . | P-value . |
|---|---|---|---|
| Agea | 1.92 | 0.78–4.72 | 0.16 |
| AF durationb | 3.74 | 1.36–10.28 | 0.011 |
| LA diameterc | 1.30 | 0.55–3.06 | 0.55 |
| DFT after ablationd | 5.54 | 2.02–15.19 | <0.001 |
| Variable . | Hazard ratio . | 95% Confidence interval . | P-value . |
|---|---|---|---|
| Agea | 1.92 | 0.78–4.72 | 0.16 |
| AF durationb | 3.74 | 1.36–10.28 | 0.011 |
| LA diameterc | 1.30 | 0.55–3.06 | 0.55 |
| DFT after ablationd | 5.54 | 2.02–15.19 | <0.001 |
aAge >65 years old, median.
bAF duration >60 months, median.
cLA diameter >47 mm, median.
dDFT after ablation >10J, median.
Multivariate analyses of the incidence of recurrent arrhythmia in patients without procedural atrial fibrillation termination
| Variable . | Hazard ratio . | 95% Confidence interval . | P-value . |
|---|---|---|---|
| Agea | 1.92 | 0.78–4.72 | 0.16 |
| AF durationb | 3.74 | 1.36–10.28 | 0.011 |
| LA diameterc | 1.30 | 0.55–3.06 | 0.55 |
| DFT after ablationd | 5.54 | 2.02–15.19 | <0.001 |
| Variable . | Hazard ratio . | 95% Confidence interval . | P-value . |
|---|---|---|---|
| Agea | 1.92 | 0.78–4.72 | 0.16 |
| AF durationb | 3.74 | 1.36–10.28 | 0.011 |
| LA diameterc | 1.30 | 0.55–3.06 | 0.55 |
| DFT after ablationd | 5.54 | 2.02–15.19 | <0.001 |
aAge >65 years old, median.
bAF duration >60 months, median.
cLA diameter >47 mm, median.
dDFT after ablation >10J, median.
Discussion
The present study showed that (i) termination of AF during ablation is associated with a good clinical outcome, (ii) patients requiring cardioversion after completion of a predetermined stepwise ablation still have a good prognosis when DFT is relatively low, and (iii) multivariate analysis showed that high DFT after ablation was a strong independent predictor of recurrent arrhythmia.
Different defibrillation threshold and catheter ablation
Although the mechanism behind successful electrical cardioversion of AF is not completely understood, previous studies have shown that DFT may be related to global organization of AF.16–19 Lower dominant fibrillatory frequencies, which may be associated with increased organization of AF and duration of the excitable gap, result in lower DFT levels.16–19 Other earlier studies have also shown that a decrease in fibrillatory frequencies and a prolongation of atrial refractoriness following the administration of antiarrhythmic drugs are related to a reduction in DFT.21–23 Different electrophysiologic substrates may determine the atrial defibrillation energy requirements. Some patients may have focal triggers with stable low frequency areas which could require low defibrillation energy. Others may have multiple wavelets and higher frequency areas making them more refractory to successful cardioversion. Our findings can be explained by these possible mechanisms allowing cardioversion of AF to sinus rhythm. Low DFT after ablation could be the consequence of an increase in AF organization as a result of catheter ablation. Circumferential PV isolation and electrogram-based ablation decrease the dominant fibrillatory frequency of persistent AF and increase AF organization,24,25 which may lead to the reduction in DFT. On the contrary, high DFT after ablation probably reflects the extent of the residual AF drivers. Of note, in this study, DFT at the repeat ablation procedure was lower than that at the first ablation procedure. This finding suggests that even if AF persisted at the end of the repeat procedure, atrial electrophysiologic substrates of these patients might successively attain higher organization level after the repeat procedure, compared with that after the first procedure.
Clinical efficacy
Catheter ablation for persistent AF is currently performed with variable outcome through use of a combination of different techniques.9–15 Procedural AF termination could be achieved in more than half of the long-standing persistent AF, and was associated with a higher arrhythmia-free rate than when AF was sustained after ablation.11–15 A prior prospective study reported an improved clinical outcome of catheter ablation using procedural termination of persistent AF as the endpoint of the ablation procedure.15 Procedural AF termination could be interpreted as an adequate elimination of the critical drivers that were maintaining the AF at the time. The clinical outcome in this study was consistent with these previous reports. We found, however, that patients with procedural AF termination had significantly smaller baseline LA size and shorter AF duration than did those without procedural AF termination. This finding was also consistent with previous studies.15,26,27 In patients with enlarged LA, procedural AF termination is difficult even after an extensive ablation, because the enlarged LA may harbour multiple re-entrant wavelets and have more severe AF burden. The problem lies in determining the procedural endpoint when AF cannot be terminated after the completion of a predetermined lesion set, especially under these circumstances. More extensive ablation to terminate AF may result in a higher possibility of complications.
Our study showed a comparable arrhythmia-free rate between patients who achieved procedural AF termination and those who underwent successful electrical cardioversion at low DFT after completion of a predetermined stepwise ablation. In addition, we found that the majority of the arrhythmia recurrence after the initial procedure was organized atrial tachycardia in these patients. This finding suggests that patients in whom DFT is low after the initial ablation procedure may achieve effective modification of the atrial electrophysiologic substrate, in which fibrillation could no longer occur. Furthermore, a markedly high success rate after repeat ablation procedure could be due to effective modification of atrial substrate that can no longer fibrillate after the first procedure and elimination of recurrent organized tachycardia achieved after the repeat ablation procedure. If procedural AF termination could not be achieved after completion of a predetermined lesion set, further extensive ablation trying to terminate AF might not seem to be warranted, especially in patients in whom AF could be successfully terminated by cardioversion at low DFT, which may indicate adequate modification of the atrial electrophysiologic substrate.
Limitations
This study was a retrospective analysis, and bias might have occurred because the measurement of DFT was not performed systematically, at least not for the purpose of DFT research. As previously described, electrical cardioversion can be performed by using a step-up and step-down defibrillation protocol that requires AF re-induction.16,17 One limitation of this study is that our cardioversion protocol using the step-up approach implies some potential problems of reproducibility. However, the underlying electrophysiologic properties are different between spontaneous persistent AF and re-induced AF or atrial tachycardia. Therefore, it may be difficult to compare DFT values of these arrhythmias. The reliability of the reproducibility of a second measurement of DFT in re-induced arrhythmia has not been established.28,29
Another potential limitation is 5 J incremental steps in cardioversion energy delivery when determining DFT. Although we found significant difference in the clinical outcome between patients with DFT of >10J and those with DFT of ≤10J by using our defibrillation protocol, further research by using much smaller increments of defibrillation energy delivery might be required to determine a more accurate cut-off point for DFT. However, it may be difficult in clinical research in human beings. To limit the number of delivered shocks in the clinical setting of this study, electrical cardioversion using the 5 J step-up approach was performed with the aim of returning the patient to sinus rhythm.
Lastly, lower DFT was significantly related to more improved clinical outcome and could be an available indicator of the endpoint of ablation for persistent AF. However, we did not perform the stepwise ablation using low DFT as a procedural endpoint. The clinical implication of DFT after ablation as a procedural endpoint requires validation in prospective studies.
Conclusions
Procedural AF termination of persistent AF is associated with a high probability of freedom from atrial arrhythmia recurrence. In addition to procedural AF termination, low DFT after ablation is likewise associated with a better clinical outcome than when high DFT is needed to restore sinus rhythm. When AF cannot terminate after the completion of a predetermined lesion set, further extensive ablation to terminate AF might be unnecessary, if the AF can be successfully terminated by electrical cardioversion at low DFT.
Conflict of interest: none declared.



