-
PDF
- Split View
-
Views
-
Cite
Cite
Danish Ali, Abdalla Alzuwam, Gerry P McCann, Martin Been, Faizel Osman, Giant cell myocarditis: an unusual presentation, EP Europace, Volume 13, Issue 12, December 2011, Pages 1793–1794, https://doi.org/10.1093/europace/eur202
Close - Share Icon Share
Abstract
Giant cell myocarditis is a rare but often devastating disease affecting young, otherwise healthy individuals. Patients often die of heart failure and ventricular arrhythmia unless cardiac transplantation is performed. Cardiac magnetic resonance imaging with or without cardiac biopsy can be helpful in making the correct diagnosis and ensuring that correct timely treatment is administered.
A 57-year-old man presented with increasing breathlessness and pre-syncope. He had recently visited Peru, and had been well with no insect bites while abroad. He had treated hypothyroidism, his only medication was thyroxine. He drank occasional alcohol and had never used illicit drugs. On examination he was haemodynamically stable with no cardiac failure, resting pulse rate 40 bpm, respiratory, abdominal, and neurological examinations were unremarkable. Resting 12-lead electrocardiogram (ECG) revealed complete heart block, right bundle branch block pattern, ST elevation in leads III, aVF, and V1, and ST depression in leads I, aVL, and V2 (Figure 1A); the latter changes resolved within 4 days. Chest X-ray and baseline bloods (renal, liver, thyroid, blood count, inflammatory markers, and autoimmune screen) were normal as was serum angiotensin-converting enzyme level; troponin T was 0.42 µg/L (normal <0.01 µg/L) at presentation, but began to fall a few days later. Initial transthoracic echocardiography revealed mild left ventricular (LV) systolic impairment (ejection fraction 40–45%) with no valvular abnormalities but subsequent echocardiography a few days later demonstrated severe global LV systolic impairment with an ejection fraction <30%; right ventricular systolic function was also impaired having been preserved on the initial echo. Coronary angiography revealed normal coronary arteries. Rhythm monitoring revealed heart block with episodes of sustained ventricular tachycardia (heat rate 180 bpm) that was associated with no syncope.
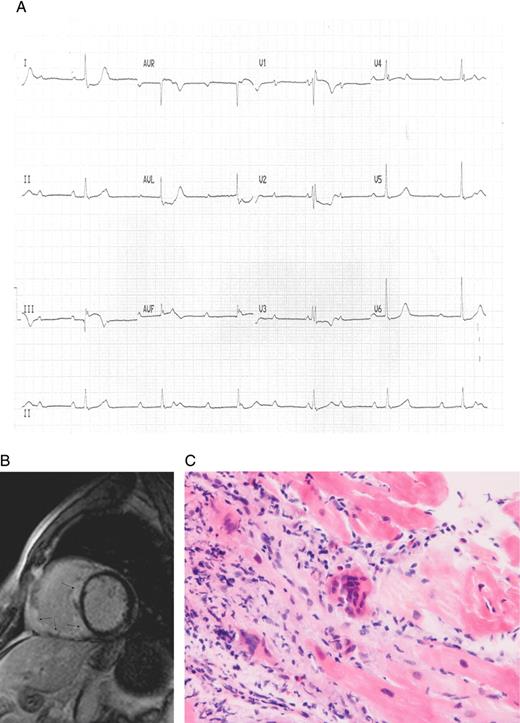
(A) Resting electrocardiogram demonstrating heart block, right bundle branch block pattern, and ST elevation in limb leads III and aVF with ST depression in leads I, aVL, and V2. (B) Cardiac magnetic resonance imaging scan showing extensive myocardial infiltration affecting most of the right ventricle and interventricular septum with abnormal late gadolinium hyper-enhancement in these regions. (C) Histology from a cardiac biopsy taken from the right ventricular septum revealing a prominent mixed inflammatory infiltrate including macrophages, giant cells, lymphocytes (mostly T cells), and eosinophils consistent with giant cell myocarditis.
A cardiac magnetic resonance imaging (MRI) scan was performed, which showed extensive infiltrative cardiomyopathy affecting most of the right ventricle (RV) and interventricular septum with extensive abnormal late gadolinium hyper-enhancement of most of the RV/septum (Figure 1B). Cardiac biopsy of the RV septum revealed prominent mixed inflammatory infiltrate including macrophages, giant cells, lymphocytes (mostly T cells), and eosinophils consistent with a diagnosis of idiopathic giant cell myocarditis (Figure 1C). The patient underwent implantation of a cardiac resynchronization therapy-defibrillator and was commenced onto an angiotensin receptor antagonist, ß-blocker, oral steroids, and cyclosporine therapy. During follow-up, he developed slow ventricular tachycardia (heart rate 110 bpm) with no haemodynamic compromise which was successfully treated with up-titration of his ß-blocker and addition of amiodarone. Biventricular systolic function remains severely impaired and he is awaiting cardiac transplantation.
Giant cell myocarditis is a rare but often devastating disease affecting young, otherwise healthy individuals. Patients often die of heart failure and ventricular arrhythmia unless cardiac transplantation is performed.1 Despite the possibility of fatal disease recurrence, transplantation is the treatment of choice for most. The rate of death or heart transplantation is ∼70% at 1 year.2 Data from observational human studies suggest that giant cell myocarditis is mediated by T lymphocytes and may respond to treatment aimed at attenuating T cell function.2 Findings from the Giant Cell Myocarditis Registry suggested that subjects who received cyclosporine in combination with steroid, azathioprine, or muromonab-CD3 have prolonged transplant-free survival (12.6 vs. 3.0 months for no immunosuppression).1 A recent prospective study of immunosuppression for giant cell myocarditis confirmed retrospective case reports that such therapy improves long-term survival.3 Additionally, withdrawal of immunosuppression can be associated with fatal recurrence. Post-transplantation survival is ∼71% at 5 years despite a 25% rate of giant cell infiltration in the donor heart.2
The presence of ST-elevation and depression on resting ECG is an unusual and novel presentation. Our patient had ST depression in leads I, aVL, and V2, and ST elevation in leads III, aVF, and V1 (which later resolved). Cardiac MRI revealed extensive infiltration in the RV and septal regions that may have accounted for the ST changes seen on ECG; this extensive infiltration may have altered repolarization characteristics of the epi/endocardium. Lead II did not have ST elevation which may have been related to the specific pattern of myocardial infiltration. There was extensive septal infiltration affecting the atrioventricular node/His-Purkinje system accounting for the conduction disturbances seen. Ventricular arrhythmias have been reported1 but brady-arrhythmias and ST changes have not.
Patients presenting with myocarditis, heart block, and/or ST changes should undergo cardiac MRI to better define cardiac anatomy. Cardiac biopsy can be helpful in making the correct diagnosis and ensuring that correct timely treatment is administered.
Conflict of interest: none declared.



