-
PDF
- Split View
-
Views
-
Cite
Cite
Sebastian Stec, Beata Zaborska, Małgorzata Sikora-Frąc, Tomasz Kryński, Piotr Kułakowski, First experience with microprobe transoesophageal echocardiography in non-sedated adults undergoing atrial fibrillation ablation: feasibility study and comparison with intracardiac echocardiography, EP Europace, Volume 13, Issue 1, January 2011, Pages 51–56, https://doi.org/10.1093/europace/euq349
Close - Share Icon Share
Abstract
Imaging of the left atrium (LA) is mandatory during catheter ablation of atrial fibrillation (AF) and may be achieved by echocardiography. The aim of the present study was to assess the feasibility of using a recently released transoesophageal echocardiography (TEE) microprobe (micro-TEE) in non-sedated adult patients undergoing AF ablation and to directly compare this new technique with intracardiac echocardiography (ICE).
The study group consisted of 12 consecutive patients (8 males, mean age 49 ± 14 years) who underwent first radiofrequency AF ablation. All patients underwent standard TEE, computed tomography, intraprocedural micro-TEE, and ICE. The easiness of introducing the microprobe in the supine position in non-sedated patients in the electrophysiology laboratory, its tolerability, and quality of obtained images were assessed using a five-point scale. There were no problems with microprobe introduction and obtaining images for a mean of 54 ± 17 min. The microprobe was significantly better tolerated than the standard TEE probe (4.3 ± 0.5 vs. 3.4 ± 0.6 points, P< 0.01). The micro-TEE was scored as significantly better than ICE in the assessment of the LA and LA appendage (LAA) anatomy and function. Both techniques were very useful in guiding transseptal puncture, although micro-TEE images were ranked higher by an echocardiographer than by an electrophysiologist (tenting 4.8 ± 0.6 vs. 4.0 ± 0.6 points, P< 0.01), whereas ICE images were ranked equally excellent by both observers.
In non-sedated patients undergoing AF ablation, the micro-TEE can be used for the assessment of the LA, LAA, and pulmonary veins anatomy as well as the guidance of transseptal puncture.
Introduction
Percutaneous catheter ablation of atrial fibrillation (AF) is a complex electrophysiological procedure, requiring several tools to understand cardiac anatomy. In addition, it requires access to the left atrium (LA) which is obtained by transseptal puncture. Intracardiac echocardiography (ICE) has been used to facilitate transseptal puncture, assess the LA and pulmonary vein (PV) anatomy, as well as monitor accuracy of ablation lesions and complications.1–3 However, ICE is an invasive tool—it requires additional venous puncture. Also, the cost of a single-use probe is not negligible.
Transoesophageal echocardiography (TEE) has been used for many years to assess LA anatomy, especially to exclude thrombus in the LA appendage (LAA), and also to facilitate transseptal puncture.4 Recently, a real-time three-dimensional TEE has been successfully used for the transseptal puncture guidance.5,6 However, it is difficult to keep in the oesophagus a standard thick TEE probe for a longer period of time in non-sedated patients due to a considerable discomfort. Thus, a search for a more tolerable tool is justified.
The aim of the present study was to assess the feasibility of using recently released multiplane TEE microprobe (micro-TEE) for delineating LA and PV anatomy as well as for the guidance of transseptal puncture in non-sedated adult patients undergoing AF ablation and to directly compare this new technique with standard ICE.
Methods
Patients
The study group consisted of 12 consecutive patients (8 males, mean age 49 ± 14 years) who underwent first radiofrequency (RF) AF ablation [PV isolation (PVI)] in our institution between 9 February and 17 March 2010. Nine patients had paroxysmal AF, whereas the remaining three persistent AF. In all patients, oral anticoagulants were stopped 2–3 days before the procedure and low-molecular-weight heparin was introduced. One to 2 days before the procedure, a computerized tomography (CT) of the LA and PV was performed in order to delineate their anatomy. The protocol of the study was approved by the local Ethics Committee and all patients gave their written, informed consent to participate in the study.
On the day of the procedure, just before transportation to the electrophysiology laboratory (EP lab), all patients underwent standard TEE to exclude thrombus in the LAA (multiplane TEE probe 6T, Vivid 4, GE Vingmed Ultrasound, Horten, Norway). Posterior pharyngeal anaesthesia was done in all cases. Probe was lubricated with surgical jelly containing lignocaine and patients were placed in the left lateral decubitus position. In two patients, light sedation with 2 mg of midazolam was also performed. After 20 min of preparation, the image acquisition took 10–20 min.
Intraprocedural transoesophageal echocardiography
Immediately after arrival to the EP lab, posterior pharyngeal anaesthesia was performed using the same jelly as during standard TEE and the patients were placed in the supine position. A very thin TEE probe (Micro TEE S8-3t, Philips Ultrasound, Bothell, WA, USA) with echocardiography system (Philips iE33, Bothell, WA, USA) was used. Properly lubricated microprobe was introduced into the oropharynx and gradually advanced into the oesophagus (no additional sedation was used). This transducer is 7.5 × 5.5 × 18.5 mm in width, height, and length and has a shaft diameter of 5.2 mm. It was developed for cardiac imaging of neonatal patients and is markedly thinner than the standard TEE probe used in adults (Figure 1). The micro-TEE is capable of delivering pulse-wave Doppler, continuous-wave Doppler, colour Doppler, XRES adaptive image processing, motion modulation, and harmonic imaging in two dimensions. The probe was introduced in the supine position to evaluate the LA and PV anatomy. Starting from four-chamber view, multiple manoeuvres (rotation, anteflexion, retraction, and advancement) were done to obtain and digitally store all transoesophageal images as well as to guide transseptal puncture. In order to obtain optimal views, standard manoeuvres, adapting the level of the probe and rotating from 0° to 180°, were done. Transgastric views were not attempted. After a single transseptal puncture was performed (we usually introduce a second sheath via the same puncture) and catheters were placed in the LA, the microprobe was removed and the ablation procedure was started.

Intracardiac echocardiography
The ICE probe (Acunav 9 F, Siemens Ag Medical Solution, Erlangen, Germany) was introduced via the left femoral vein, positioned in the right atrium (to obtain the so-called ‘home view’) and connected to the portable echocardiography system (Cypress, Siemens Ag Medical Solution). In order to obtain the LA, LAA, and PV images, a counterclockwise torque and various probe angulations were performed.
Other equipment
In all patients, four punctures in the femoral veins were performed (two each side) for introducing sheaths for 4 mm ablation catheter (Navistar, Biosense Webster, Inc., Diamond Bar, CA, USA), circular mapping catheter (Lasso, Biosense Webster, Inc.), and steerable decapolar catheter (Biosense Webster, Inc.) introduced into the coronary sinus for pacing and for the ICE probe. The ablation and diagnostic catheters were connected to the electroanatomical system (CARTO XP, Biosense Webster, Inc.) and electrophysiological system (The Bard LabSystem, PRO EP Recording System, Bard, Inc., Lowell, MA, USA).
Assessment of micro-transoesophageal echocardiography feasibility
The easiness of introducing the microprobe in the supine position in EP lab was assessed by echocardiographers who were responsible for TEE imaging (B.Z. and M.S.-F.), using a five-point scale (5, easy without any problem; 4, slight problems during introducing the probe; 3, significant problems; 2, significant problems requiring more than one attempt to introduce the probe; 1, failure of introducing the probe). The duration of the microprobe stay in the oesophagus was measured. After removal of the microprobe, a patient was asked about tolerability of the probe, using a five-point scale (5, no or minimal discomfort; 4, slight discomfort; 3, significant discomfort; 2, major discomfort; 1, major discomfort necessitating premature probe removal).
The same point score was used to assess patient's tolerability of the standard TEE performed before the procedure and then both scores were compared in order to compare tolerability of the two techniques.
Comparison between micro-transoesophageal echocardiography and intracardiac echocardiography in the left atrial and pulmonary vein anatomy assessment
When both the micro-TEE and ICE probes were in place, together with two long sheaths in the right atrium and a diagnostic catheter in the coronary sinus, the LA cavity, the LAA, and PV were visualized using these two techniques. It had to be done step by step, freezing and unfreezing each of the echocardiography systems, because of the image distortion and artefacts when they were used simultaneously. First, LA cavity was inspected for the presence of spontaneous contrast and a possible thrombus, and next the LAA was examined. Both the structure and the function (flow) of the LAA were assessed. This was followed by the assessment of the left and right PVs (both diameter and flow).
Both techniques were also compared regarding the visualization of interatrial septum, transseptal needle (Brockenbrough needle, St Jude Medical, St Paul, MN, USA), septum tenting, entering the LA, bubbles in the LA produced by saline injection via a transseptal sheath, and two guiding wires inserted in the left superior PV (LSPV) and LAA or right superior PV (RSPV). At the very moment of the puncture, we used ICE since electrophysiologists are more familiar with the images produced by ICE. Typical radiographic image of the micro-TEE and ICE probes during transseptal puncture is shown in Figure 2.
![Antero-posterior X-ray view obtained after introducing micro-TEE probe and intracardiac equipment used during RF ablation. From the right femoral venous approach, transseptal sheaths for circular mapping catheter [located into the left superior pulmonary vein (LSPV)] and mapping catheter [located deeply into the right superior pulmonary vein (RSPV)] were introduced. From the left femoral venous approach, a decapolar catheter was inserted into coronary sinus and the ICE probe was advanced in the right atrium. Microprobe is located in the middle of the oesophagus.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/europace/13/1/10.1093_europace_euq349/3/m_euq34902.jpeg?Expires=1748791560&Signature=Cy59vbInNaRXGUJy3RKawNw8ezTpfxJx~6SCZipvGO9tOu8PooduwpjDiMnMueBVK0zXOAUxOOHdWy401SMBgP3luH4laOsGXF-ST8yJyrbjYEOFNr7ch2559YKywA0KZpUmXh5120OiA4BhsBm9gOPFoxGEnp-NealpQZQVnh~HnJxR1zWi6Q0x2GFpcoJGS87PKlvQ-zyT9HIglzhXsCkujx-fIITl4MRX0-APUZtR9zzqFn1HcQRgFXR52TN5RdbDcRwN7LlIdrLBbJah~Q0LqKddRLEmHAx-RiLY9eDldYItpWiHstbwQ2PsK6LUt-JtMwCPP6z46~uM9UpP~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Antero-posterior X-ray view obtained after introducing micro-TEE probe and intracardiac equipment used during RF ablation. From the right femoral venous approach, transseptal sheaths for circular mapping catheter [located into the left superior pulmonary vein (LSPV)] and mapping catheter [located deeply into the right superior pulmonary vein (RSPV)] were introduced. From the left femoral venous approach, a decapolar catheter was inserted into coronary sinus and the ICE probe was advanced in the right atrium. Microprobe is located in the middle of the oesophagus.
All images were inspected first online during the procedure independently by an echocardiographer and an electrophysiologist and were stored digitally. The number of points granted for each image was then calculated (5, excellent visualization of the structure or its function; 4, good; 3, medium quality; 2, poor quality; 1, very poor quality). Next, the images were analysed by the echocardiographer and the electrophysiologist, and a joint agreement was obtained. In the case of discrepant results, both scores were included in the analysis. After this assessment, the microprobe was removed from the oesophagus and total duration of the microprobe stay in the oesophagus was recorded.
Statistical analysis
The results are presented as mean ± standard deviation or numbers and percentages. The differences in the analysed parameters were examined using a two-tailed paired t-test for continuous parameters. A P-value <0.05 was considered significant.
Results
All consecutive 12 patients scheduled for AF ablation had clear CT and pre-procedural standard TEE and agreed to undergo additional intraprocedural micro-TEE evaluation according to the study protocol. There were no problems with the microprobe insertion into the oesophagus in the supine position—the echocardiographer rated the easiness of intubation as 5 points in 10 patients and 4 points in 2 patients (mean 4.8 ± 0.4 points). The microprobe remained in the oesophagus for a mean of 54.4 ± 17.0 min (range: from 35 to 80 min). There were no chocking, vomiting reflexes, overproduction of saliva, severe oesophageal pain, or injury during and after micro-TEE examination.
All patients tolerated very well the microprobe (four patients: 5 points and eight patients: 4 points) and there was no need for premature removal of the probe. The microprobe was significantly better tolerated than the standard TEE probe (six patients: 4 points, five patients: 3 points, and one patient: 2 points) (4.3 ± 0.5 vs. 3.4 ± 0.6 points, P< 0.01).
The comparison between the quality of the micro-TEE and ICE images of LA and PV is presented in Table 1. The micro-TEE technique was scored by the investigators as significantly better than ICE in the assessment of the LA and LAA anatomy and tended to be better in the LAA function evaluation. Out of the four PVs (no patient had additional PV), only LSPV was properly visualized by both techniques in each patient. The scores for the LSPV visualization tended to be higher for ICE (NS). The left inferior was visualized by micro-TEE in 8 patients vs. 11 patients by ICE, the RSPV in 10 vs. 6 patients, and the right inferior PV in 6 vs. 5 patients, respectively. There were no significant differences between the echocardiographer and the electrophysiologist in the assessment of the quality of LA and PV images using the two techniques. Representative examples of the LAA and LSPV visualization using both techniques are shown in Figure 3.
Comparison of the quality of micro-transoesophageal echocardiography and intracardiac echocardiography images of the left atrium and pulmonary veins
| Parameter . | Micro-TEE . | ICE . | P-value . |
|---|---|---|---|
| LA visualization | 4.9 ± 0.28 | 4.25 ± 0.76 | 0.025 |
| LAA anatomy | 4.4 ± 0.9 | 3.3 ± 1.2 | 0.04 |
| LAA function | 4.4 ± 0.5 | 3.8 ± 1.1 | NS |
| LSPV anatomy | 4.3 ± 1.2 | 4.5 ± 0.7 | NS |
| LSPV flow | 4.4 ± 1.2 | 4.5 ± 0.7 | NS |
| Parameter . | Micro-TEE . | ICE . | P-value . |
|---|---|---|---|
| LA visualization | 4.9 ± 0.28 | 4.25 ± 0.76 | 0.025 |
| LAA anatomy | 4.4 ± 0.9 | 3.3 ± 1.2 | 0.04 |
| LAA function | 4.4 ± 0.5 | 3.8 ± 1.1 | NS |
| LSPV anatomy | 4.3 ± 1.2 | 4.5 ± 0.7 | NS |
| LSPV flow | 4.4 ± 1.2 | 4.5 ± 0.7 | NS |
The mean values of the point score are shown (from 5, excellent to 1, very poor).
Comparison of the quality of micro-transoesophageal echocardiography and intracardiac echocardiography images of the left atrium and pulmonary veins
| Parameter . | Micro-TEE . | ICE . | P-value . |
|---|---|---|---|
| LA visualization | 4.9 ± 0.28 | 4.25 ± 0.76 | 0.025 |
| LAA anatomy | 4.4 ± 0.9 | 3.3 ± 1.2 | 0.04 |
| LAA function | 4.4 ± 0.5 | 3.8 ± 1.1 | NS |
| LSPV anatomy | 4.3 ± 1.2 | 4.5 ± 0.7 | NS |
| LSPV flow | 4.4 ± 1.2 | 4.5 ± 0.7 | NS |
| Parameter . | Micro-TEE . | ICE . | P-value . |
|---|---|---|---|
| LA visualization | 4.9 ± 0.28 | 4.25 ± 0.76 | 0.025 |
| LAA anatomy | 4.4 ± 0.9 | 3.3 ± 1.2 | 0.04 |
| LAA function | 4.4 ± 0.5 | 3.8 ± 1.1 | NS |
| LSPV anatomy | 4.3 ± 1.2 | 4.5 ± 0.7 | NS |
| LSPV flow | 4.4 ± 1.2 | 4.5 ± 0.7 | NS |
The mean values of the point score are shown (from 5, excellent to 1, very poor).
Both the micro-TEE and ICE were useful in guiding transseptal puncture; however, there were significant differences between the echocardiographer and the electrophysiologist in the quality of septum visualization and tenting (Table 2). According to the echocardiographer, both techniques were equally very good, whereas electrophysiologist ranked higher the ICE vs. micro-TEE images (Table 2).
Comparison of the quality of images before, during, and after transseptal puncture using micro-transoesophageal echocardiography and intracardiac echocardiography assessed by an echocardiographer and an electrophysiologist
| Parameter . | Micro-TEE . | P-value . | ICE . | P-value . | ||
|---|---|---|---|---|---|---|
| . | Echo . | EP . | . | Echo . | EP . | . |
| Interatrial septum | 4.8 ± 0.6 | 4.2 ± 0.4 | <0.01 | 4.8 ± 0.4 | 4.8 ± 0.4* | NS |
| Tenting | 4.8 ± 0.6 | 4.0 ± 0.6 | <0.01 | 4.8 ± 0.4 | 4.8 ± 0.4* | NS |
| Saline bubbles in LA | 4.9 ± 0.3 | 4.9 ± 0.3 | NS | 5.0 ± 0 | 5.0 ± 0 | NS |
| Parameter . | Micro-TEE . | P-value . | ICE . | P-value . | ||
|---|---|---|---|---|---|---|
| . | Echo . | EP . | . | Echo . | EP . | . |
| Interatrial septum | 4.8 ± 0.6 | 4.2 ± 0.4 | <0.01 | 4.8 ± 0.4 | 4.8 ± 0.4* | NS |
| Tenting | 4.8 ± 0.6 | 4.0 ± 0.6 | <0.01 | 4.8 ± 0.4 | 4.8 ± 0.4* | NS |
| Saline bubbles in LA | 4.9 ± 0.3 | 4.9 ± 0.3 | NS | 5.0 ± 0 | 5.0 ± 0 | NS |
The mean values of the point score are shown (from 5, excellent to 1, very poor). Echo, echocardiographer; EP, electrophysiologist.
*P < 0.01, Echo vs. EP.
Comparison of the quality of images before, during, and after transseptal puncture using micro-transoesophageal echocardiography and intracardiac echocardiography assessed by an echocardiographer and an electrophysiologist
| Parameter . | Micro-TEE . | P-value . | ICE . | P-value . | ||
|---|---|---|---|---|---|---|
| . | Echo . | EP . | . | Echo . | EP . | . |
| Interatrial septum | 4.8 ± 0.6 | 4.2 ± 0.4 | <0.01 | 4.8 ± 0.4 | 4.8 ± 0.4* | NS |
| Tenting | 4.8 ± 0.6 | 4.0 ± 0.6 | <0.01 | 4.8 ± 0.4 | 4.8 ± 0.4* | NS |
| Saline bubbles in LA | 4.9 ± 0.3 | 4.9 ± 0.3 | NS | 5.0 ± 0 | 5.0 ± 0 | NS |
| Parameter . | Micro-TEE . | P-value . | ICE . | P-value . | ||
|---|---|---|---|---|---|---|
| . | Echo . | EP . | . | Echo . | EP . | . |
| Interatrial septum | 4.8 ± 0.6 | 4.2 ± 0.4 | <0.01 | 4.8 ± 0.4 | 4.8 ± 0.4* | NS |
| Tenting | 4.8 ± 0.6 | 4.0 ± 0.6 | <0.01 | 4.8 ± 0.4 | 4.8 ± 0.4* | NS |
| Saline bubbles in LA | 4.9 ± 0.3 | 4.9 ± 0.3 | NS | 5.0 ± 0 | 5.0 ± 0 | NS |
The mean values of the point score are shown (from 5, excellent to 1, very poor). Echo, echocardiographer; EP, electrophysiologist.
*P < 0.01, Echo vs. EP.
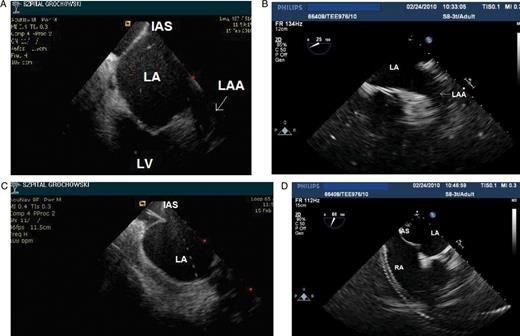
Left atrial appendage (arrows) visualization by using ICE (A) and micro-TEE (B) prior to transseptal puncture. Visualization of tenting of the interatrial septum by using ICE (C) and micro-TEE (D).
There were three patients with a small fresh thrombus (two in the right atrium and one in the LA), all localized near the septum. All thrombuses were detected just before or at the time of transseptal puncture, before heparin injection. In two patients, the thrombus was visible only using ICE, and in the third patient using both techniques, however, better using ICE than micro-TEE (5 vs. 3 points). The thrombus resolved after heparin infusion.
Following the removal of the microprobe, all 12 patients underwent PVIs with guidance and monitoring by ICE. All 12 procedures were performed without any complications and PVs were isolated in all patients.
Discussion
Our pilot study showed that a new thin microprobe is very well tolerated by an adult patient even for a prolonged period of time (a mean of 54 min), provides good-quality images of the LA and PV, and is useful for the guidance of transseptal puncture. Therefore, it may be suggested that this new tool could be used during preparatory stages of AF ablation.
To date, only standard, relatively thick TEE probes have been successfully used for the assessment of LA structures and transseptal puncture guidance.4,7 This is a well-established tool for this purpose; however, a patient needs to be anaesthesized or at least has to receive very deep sedation. Since in many centres, AF ablation is performed in patients receiving only a light sedation, this technique is not feasible. Moreover, the use of light sedation has been shown to be associated with a lower rate of oesophageal complications when compared with general anaesthesia.8
Our results documented the usefulness of micro-TEE in the assessment of the LA anatomy and PV, and possibly for the inspection of the LAA for the presence of thrombus. Our preliminary findings suggest that this technique could be useful for this purpose and is probably more accurate than ICE. The ICE is not regarded as a gold standard for the detection of LAA thrombus mainly because standard right atrial views do not provide adequate visualization of the LAA. Specifically, in five patients, problems occurred with imaging of the whole LAA, missing the most distal part of the LAA. It has been recently suggested in an animal experiment that the detection of the LAA thrombus by ICE may be improved by imaging the LAA from the pulmonary artery.9 However, in our study, we used only standard ICE views form the right atrium. In addition, no patient in our cohort had the LAA thrombus which was ruled out by standard pre-procedural TEE and CT; therefore, the value of the microprobe in detecting a thrombus in the LAA could not be established in our study.
Another potential use of the new microprobe could be the guiding of transseptal puncture. We showed that the quality of images assessed by an echocardiographer was not inferior to that obtained using ICE which is a well-accepted tool for transseptal puncture guidance. The electrophysiologist ranked ICE slightly higher than micro-TEE. This may be due to the fact that electrophysiologists were more familiar with ICE than TEE images. It also suggests that the presence of an experienced echocardiographer is recommended when using a new TEE technique, at least during the initial phase of introducing this procedure.
The fact that patients tolerated better the micro-TEE than a standard TEE probe is not surprising. It further suggests that the prolonged intraprocedural use of micro-TEE in non-sedated adults is feasible not only for point-by-point RF ablation techniques but may be possibly useful for multielectrode RF catheters, cryoballons, and other cardiovascular intervention such as LAA or atrial septal defect-closing device implantation.
Another potential use of the microprobe would be further assistance during the AF ablation procedure itself—guiding PVI and early detection of procedure-related complications. Actually, this was our original plan when we designed the study. However, we abandoned this idea right away during the first session when we realized that imaging with TEE transducer is associated with a rise in the probe temperature, and subsequently, probably in the oesophageal temperature. This rise was rather small when imaging the structures (up to 38.1°C); however, it was much higher (above 39°C) when using colour Doppler to measure the flow. Since any rise in the oesophageal temperature may be dangerous during AF ablation, we immediately abandoned the idea of ablation monitoring by using micro-TEE and decided to limit the study to the time point of transseptal puncture. Further investigation on temperature rise and potential harm from TEE probe to the oesophagus should be verified before permanent monitoring of RF ablation, especially when performed in the posterior wall of LA.
In summary, in non-sedated patients undergoing AF ablation, the micro-TEE could be used for the assessment of the LA and LAA anatomy, delineation of PV anatomy, and for the guidance of transseptal puncture. These preliminary findings have to be confirmed in larger studies.
Limitations of the study
The study has several important limitations. First, the number of patients was very small and our results must be treated as preliminary. Secondly, we have never used before the microprobe; thus, our results may be different after a learning curve for this new TEE tool is completed. Thirdly, we did not use other than right atrial views when performing ICE which may explain a relatively low number of properly visualized right PV and the whole cavity of LAA. Moreover, the analysis of the images was not performed blindly. Lastly, the presence of the echocardiographer during the ablation procedure raised additional costs.
Conflict of interest: none declared.
Funding
This work was supported by the research grant from the Postgraduate Medical School, Warsaw, Poland, 501-1-10-40-10.



