-
PDF
- Split View
-
Views
-
Cite
Cite
Rafael Barba-Pichardo, Pablo Moriña-Vázquez, Juan M. Fernández-Gómez, José Venegas-Gamero, Manuel Herrera-Carranza, Permanent His-bundle pacing: seeking physiological ventricular pacing, EP Europace, Volume 12, Issue 4, April 2010, Pages 527–533, https://doi.org/10.1093/europace/euq038
Close - Share Icon Share
Abstract
Right ventricular apical pacing can have deleterious effects and the His bundle has been widely reported to be an alternative site. This paper presents our experience with permanent His-bundle pacing (HBP).
Patients referred for pacemaker implants (regardless of block type) were screened to determine if temporary HBP corrected conduction dysfunctions (threshold ≤2.5 V for 1 ms) and provided infra-Hisian 1:1 conduction of at least 120 s/m. Of the 182 patients selected, HBP corrected conduction dysfunctions in 133 (73%) patients, 42 (32%) of whom were rejected for the permanent procedure due to high thresholds. His-bundle lead implantation was attempted in the remaining 91 patients and was successful in 59 (65% of all attempts, 44% of all possible cases).
In some patients, permanent HBP may be an alternative to right ventricular apical pacing.
Introduction
The right ventricular apex is widely recognized as not ideal for permanent pacing of the heart 1 because of the potential for ventricular function deterioration, 2 , 3 mitral valve dysfunction, 4 proarrhythmic effects, 5 and a higher mortality in certain patient groups. 6 An incorrect pacing mode may be technologically and economically wasteful, and some studies suggest that the DDD mode is not always superior to the VVI mode in terms of quality of life, vascular complications, or survival. 7–9
The outflow tract has been used as the alternative to the apex; however, the results have been discouraging. 10
Because His-bundle pacing (HBP) produces ventricular contraction via the specific conduction system, it does not induce interventricular or intraventricular asynchrony 11 or trigger the myocardial perfusion disorders described with right ventricular apical pacing. 12 Moreover, if DDD units are used, atrioventricular (AV) synchrony is recovered physiologically. His-bundle pacing is sufficiently well-documented in cases of supra-Hisian blocks. 13–16 Surprisingly, HBP can also correct many conduction disturbances usually considered to be infra-Hisian and, therefore, can be used in selected cases under such circumstances. 17–19
The purpose of this paper is to report on the success rates and outcomes obtained in our experience with HBP.
Method
Patient selection
From January 2007 to May 2008, patients were alternately selected (i.e. one out of two, in chronological order) for HBP from among those referred for pacemaker implantation regardless of block type. In total, 182 of the 364 were chosen. Average patient age was 74 (SD 9.8) years.
Of those 182 patients, only patients who met the following inclusion criteria were considered candidates for permanent HBP: Some type of AV block with narrow QRS morphology was observed in 84 of the 182 patients; the remaining 98 patients had blocks with a wide QRS complex (QRS wider than 130 ms). Of these patients, eight had an indication of resynchronization with built-in defibrillator; two of these had failed prior attempts via coronary sinus, hence they were included for HBP.
Indication of permanent pacing for AV conduction disturbance or left ventricular resynchronization, 20 , 21 not possible via the coronary sinus.
Potential for elimination of AV block or bundle-branch block by HBP, leading to a narrow QRS complex (≤120 ms).
Maximum Hisian capture threshold of 2.5 V/1 ms (without extracting the fixation helix).
1:1 His-ventricular conduction at a minimum pacing rate of 120 b.p.m.
All procedures were performed in the electrophysiology laboratory. For temporary pacing, as well as permanent HBP when applicable, a 52-cm Tendril Model 1488T and 1788 TC active-fixation leads (St Jude, Sylmar, CA, USA) were used. All details of the His-bundle lead implantation have been previously described. 14 , 17 , 18 Briefly, the lead—connected to a polygraph—is advanced to the His area guided with a preformed stylet. Once the His deflection is recorded, temporary pacing is initiated; if the His bundle is captured with a threshold lower than 2.5 V at 1 ms, and the AV conduction disturbance disappears, then the helix is extracted while a counterclockwise torque is maintained in the stylet. Finally, the stylet is withdrawn and the lead stability is checked by forcing the lead curve. We spent no more than 60 minutes, or 20 minutes of fluoroscopy time, to implant the His-bundle lead. After this time, the procedure was abandoned and considered an unsuccessful attempt. In the case of patients implanted with defibrillators, ventricular fibrillation was then induced, while the defibrillation shock was programmed at maximum energy.
The criteria proposed by Cantu et al . 22 were used to classify the different patterns of HBP.
All patients were thoroughly informed and provided written informed consent. The study was approved by the local Ethics Committee.
Additional lead, devices employed, and programming
(A) In the presence of conduction disturbances traditionally considered to be infra-Hisian, whether paroxysmal or permanent, a second ventricular lead was implanted in the outflow tract or right ventricular apex. The final results were as follows:
In sinus rhythm, a third lead was placed in the right atrium and all three leads were connected to a Frontier II 5596 triple-chamber pacemaker (St Jude Medical) or, when applicable, to a resynchronizer with a built-in defibrillator, Atlas + HF V-341 (St Jude Medical) ( Figure 1 ).
The His-bundle lead was attached to the left ventricular channel ( Figure 2 A ), and the device was programmed in DDD mode with the left ventricle (His-bundle lead) as the first paced chamber and the VV interval was programmed at 60 ms ( Figure 2 B ).
Sensing was assigned to the right ventricular channel.
In the case of atrial fibrillation, a dual-chamber Kappa 900 pacemaker (Medtronic, Minneapolis, MN, USA) was used, connecting the His-bundle lead to the atrial port and the right ventricular lead to the respective ventricular port. A 60-ms AV interval was programmed. To prevent the inhibition of HBP by atrial fibrillation waves, the atrial channel was set to minimum sensitivity or programmed in DVI mode.
With the VV or AV delay programmed for sinus rhythm or atrial fibrillation, respectively, the right ventricular pulse falls within a refractory ventricular period, without inducing electromechanical activity, but would do so if the HBP eventually fails, serving as a safety pulse ( Figure 2 B ).
(B) In the presence of an AV conduction disturbance with a narrow QRS complex, no additional lead was implanted in the right ventricle.
In sinus rhythm, the Kappa 900 dual-chamber device was implanted.
If atrial fibrillation was present, an Insignia I AVT SR single-chamber pacemaker (Boston Scientific, St Paul, MN, USA) was used.
In both cases, the His-bundle lead was connected to the ventricular output, with the sensitivity programmed to the lowest level, in order to prevent inhibitions by the atrial waves ( Figure 1 B ).
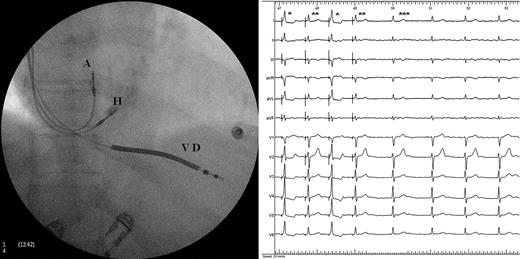
Posteroanterior radiograph of a defibrillator with His-bundle pacing resynchronization. Left Panel : A, lead in the right atrial appendage; H, lead positioned on the His bundle; VD, lead on the right ventricular apex. Right Panel : ECG in atrial fibrillation. QRS complexes marked with a single asterisk are fusion complexes, double asterisks represent pure His stimulations, and triple asterisks represent native QRS.
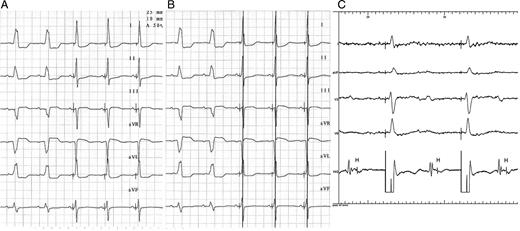
( A ) ECG in sinus rhythm with left branch block, treated with His-bundle pacing pacemaker in DDD mode. Disappearance of left block with latency and QRS normalization can be observed. ( B ) Same ECG as in ( A ), with 60-ms activation following the His-bundle pacing, right ventricular pacing; the pacing spike is seen to fall onto the R-wave. ( C ) Complete infra-Hisian block, treated with His-bundle pacing pacemaker in VVI mode; the induced QRS are of normal morphology and duration and show latency by pure His-bundle capture.
Patient follow-up consisted of clinical evaluation and monitoring His-bundle thresholds at 1 week, 1 month and 3 months after the implant. The left ventricular ejection fraction (LVEF) was assessed by echocardiography immediately before implantation and at 3 months using the biplane Simpson's method. The AV delay was programmed with echocardiographic guidance to separate A- and E-waves in mitral flow tracing, at 60 b.p.m. Results were compared using the Student's t -test.
Results
Of the 182 patients selected, 133 met the first two inclusion criteria and were considered possible candidates for permanent HBP; 91 of these patients met Criteria 3 and 4 and were therefore selected for His-bundle lead implantation, which was achieved in 59 (64.8% of all the cases in which HBP was attempted), totalling 44.3% of all possible cases ( Table 1 ).
| January 2007 to May 2008 (182 patients) . | |
|---|---|
| Block corrected with HBP | 133 (73%) |
| Block not corrected with HBP | 39 |
| Not recorded, His not captured | 10 |
| Acute HBP threshold for 1 ms | 2.2 ± 1.1 V |
| Rejected due to threshold >2.5 V | 42 (32%) |
| Accepted for electrode implant | 91 |
| Successful fixation | 59 (65%) |
| ‘ R ’ amplitude | 6.6 ± 1.5 mV |
| Fixation failures | 32 (35%) |
| Success rate for total possible implants | 44% |
| Acute HBP threshold in implanted patients (1 ms) | 1.5 ± 0.8 V |
| Impedance | 331 ± 41 Ω |
| Pure/fused HBP | 22 (37.2%)/37 (62.8%) |
| EF (%) | 52 ± 10* |
| Follow-up (at 3 months) | |
| Threshold for 0.5 ms/impedance | 1.3 ± 0.9 V, 335 ± 29 Ω |
| ‘ R ’ amplitude | 5.1 ± 1.6 mV |
| Loss of capture/dislodgement | 1/2 |
| EF (%) | 57 ± 6* |
| January 2007 to May 2008 (182 patients) . | |
|---|---|
| Block corrected with HBP | 133 (73%) |
| Block not corrected with HBP | 39 |
| Not recorded, His not captured | 10 |
| Acute HBP threshold for 1 ms | 2.2 ± 1.1 V |
| Rejected due to threshold >2.5 V | 42 (32%) |
| Accepted for electrode implant | 91 |
| Successful fixation | 59 (65%) |
| ‘ R ’ amplitude | 6.6 ± 1.5 mV |
| Fixation failures | 32 (35%) |
| Success rate for total possible implants | 44% |
| Acute HBP threshold in implanted patients (1 ms) | 1.5 ± 0.8 V |
| Impedance | 331 ± 41 Ω |
| Pure/fused HBP | 22 (37.2%)/37 (62.8%) |
| EF (%) | 52 ± 10* |
| Follow-up (at 3 months) | |
| Threshold for 0.5 ms/impedance | 1.3 ± 0.9 V, 335 ± 29 Ω |
| ‘ R ’ amplitude | 5.1 ± 1.6 mV |
| Loss of capture/dislodgement | 1/2 |
| EF (%) | 57 ± 6* |
EF, ejection fraction.
Values are means ± DS.
* P < 0.01.
| January 2007 to May 2008 (182 patients) . | |
|---|---|
| Block corrected with HBP | 133 (73%) |
| Block not corrected with HBP | 39 |
| Not recorded, His not captured | 10 |
| Acute HBP threshold for 1 ms | 2.2 ± 1.1 V |
| Rejected due to threshold >2.5 V | 42 (32%) |
| Accepted for electrode implant | 91 |
| Successful fixation | 59 (65%) |
| ‘ R ’ amplitude | 6.6 ± 1.5 mV |
| Fixation failures | 32 (35%) |
| Success rate for total possible implants | 44% |
| Acute HBP threshold in implanted patients (1 ms) | 1.5 ± 0.8 V |
| Impedance | 331 ± 41 Ω |
| Pure/fused HBP | 22 (37.2%)/37 (62.8%) |
| EF (%) | 52 ± 10* |
| Follow-up (at 3 months) | |
| Threshold for 0.5 ms/impedance | 1.3 ± 0.9 V, 335 ± 29 Ω |
| ‘ R ’ amplitude | 5.1 ± 1.6 mV |
| Loss of capture/dislodgement | 1/2 |
| EF (%) | 57 ± 6* |
| January 2007 to May 2008 (182 patients) . | |
|---|---|
| Block corrected with HBP | 133 (73%) |
| Block not corrected with HBP | 39 |
| Not recorded, His not captured | 10 |
| Acute HBP threshold for 1 ms | 2.2 ± 1.1 V |
| Rejected due to threshold >2.5 V | 42 (32%) |
| Accepted for electrode implant | 91 |
| Successful fixation | 59 (65%) |
| ‘ R ’ amplitude | 6.6 ± 1.5 mV |
| Fixation failures | 32 (35%) |
| Success rate for total possible implants | 44% |
| Acute HBP threshold in implanted patients (1 ms) | 1.5 ± 0.8 V |
| Impedance | 331 ± 41 Ω |
| Pure/fused HBP | 22 (37.2%)/37 (62.8%) |
| EF (%) | 52 ± 10* |
| Follow-up (at 3 months) | |
| Threshold for 0.5 ms/impedance | 1.3 ± 0.9 V, 335 ± 29 Ω |
| ‘ R ’ amplitude | 5.1 ± 1.6 mV |
| Loss of capture/dislodgement | 1/2 |
| EF (%) | 57 ± 6* |
EF, ejection fraction.
Values are means ± DS.
* P < 0.01.
His-bundle pacing corrects virtually all AV blocks with a narrow QRS complex (97%) and about half with a wide QRS complex (52%) ( Table 2 ).
| January 2007 to May 2008 (182 patients) . | Narrow QRS blocks 84 P . | Wide QRS Blocks 98 P . |
|---|---|---|
| Block corrected | 82 (97.6%) | 51 (52%), P < 0.01 |
| Block not corrected | 0 | 39 (39.8%) |
| Not recorded, His not captured | 2 (2.4%) | 8 (8.2%) |
| Acute threshold for 1 ms | 2.1 ± 0.9 V | 2.6 ± 1.1 V, P < 0.05 |
| Rejected due to threshold >2.5 V | 17 (21%) | 25 (49%), P < 0.01 |
| Accepted for electrode implant | 65 | 26 |
| Successful fixation | 44 (67.7%) | 15 (57%) |
| ‘ R ’ amplitude | 8 ± 2.5 mV | 5 ± 1.8 mV, P < 0.01 |
| Success rate for total possible implants | 53% | 29%, P < 0.01 |
| Acute threshold in implanted patients (1 ms) | 1.4 ± 0.6 V | 1.9 ± 1.2 V, NS |
| Impedance | 334 ± 56 Ω | 328 ± 38 Ω, NS |
| Pattern of capture | Pattern I, 18 | Pattern A, 4 |
| Pattern II, 26 | Pattern B, 11 | |
| EF (%) | 57 ± 8* | 37 ± 4** |
| Follow-up (at 3 months) | ||
| Threshold for 0.5 ms | 1.9 ± 0.8 V, | 2.4 ± 1.4 V, NS |
| Impedance | 350 ± 22 Ω | 322 ± 43 Ω, NS |
| ‘ R ’ amplitude | 5.6 ± 1.8 mV | 4.4±1.1 mV, NS |
| Lost of capture/ dislodgement | 0/2 | 1/0 |
| EF (%) | 62 ± 6* | 42 ± 5** |
| January 2007 to May 2008 (182 patients) . | Narrow QRS blocks 84 P . | Wide QRS Blocks 98 P . |
|---|---|---|
| Block corrected | 82 (97.6%) | 51 (52%), P < 0.01 |
| Block not corrected | 0 | 39 (39.8%) |
| Not recorded, His not captured | 2 (2.4%) | 8 (8.2%) |
| Acute threshold for 1 ms | 2.1 ± 0.9 V | 2.6 ± 1.1 V, P < 0.05 |
| Rejected due to threshold >2.5 V | 17 (21%) | 25 (49%), P < 0.01 |
| Accepted for electrode implant | 65 | 26 |
| Successful fixation | 44 (67.7%) | 15 (57%) |
| ‘ R ’ amplitude | 8 ± 2.5 mV | 5 ± 1.8 mV, P < 0.01 |
| Success rate for total possible implants | 53% | 29%, P < 0.01 |
| Acute threshold in implanted patients (1 ms) | 1.4 ± 0.6 V | 1.9 ± 1.2 V, NS |
| Impedance | 334 ± 56 Ω | 328 ± 38 Ω, NS |
| Pattern of capture | Pattern I, 18 | Pattern A, 4 |
| Pattern II, 26 | Pattern B, 11 | |
| EF (%) | 57 ± 8* | 37 ± 4** |
| Follow-up (at 3 months) | ||
| Threshold for 0.5 ms | 1.9 ± 0.8 V, | 2.4 ± 1.4 V, NS |
| Impedance | 350 ± 22 Ω | 322 ± 43 Ω, NS |
| ‘ R ’ amplitude | 5.6 ± 1.8 mV | 4.4±1.1 mV, NS |
| Lost of capture/ dislodgement | 0/2 | 1/0 |
| EF (%) | 62 ± 6* | 42 ± 5** |
EF, ejection fraction. Values are means ±SD; (* P <0.01); (** P <0.01).
| January 2007 to May 2008 (182 patients) . | Narrow QRS blocks 84 P . | Wide QRS Blocks 98 P . |
|---|---|---|
| Block corrected | 82 (97.6%) | 51 (52%), P < 0.01 |
| Block not corrected | 0 | 39 (39.8%) |
| Not recorded, His not captured | 2 (2.4%) | 8 (8.2%) |
| Acute threshold for 1 ms | 2.1 ± 0.9 V | 2.6 ± 1.1 V, P < 0.05 |
| Rejected due to threshold >2.5 V | 17 (21%) | 25 (49%), P < 0.01 |
| Accepted for electrode implant | 65 | 26 |
| Successful fixation | 44 (67.7%) | 15 (57%) |
| ‘ R ’ amplitude | 8 ± 2.5 mV | 5 ± 1.8 mV, P < 0.01 |
| Success rate for total possible implants | 53% | 29%, P < 0.01 |
| Acute threshold in implanted patients (1 ms) | 1.4 ± 0.6 V | 1.9 ± 1.2 V, NS |
| Impedance | 334 ± 56 Ω | 328 ± 38 Ω, NS |
| Pattern of capture | Pattern I, 18 | Pattern A, 4 |
| Pattern II, 26 | Pattern B, 11 | |
| EF (%) | 57 ± 8* | 37 ± 4** |
| Follow-up (at 3 months) | ||
| Threshold for 0.5 ms | 1.9 ± 0.8 V, | 2.4 ± 1.4 V, NS |
| Impedance | 350 ± 22 Ω | 322 ± 43 Ω, NS |
| ‘ R ’ amplitude | 5.6 ± 1.8 mV | 4.4±1.1 mV, NS |
| Lost of capture/ dislodgement | 0/2 | 1/0 |
| EF (%) | 62 ± 6* | 42 ± 5** |
| January 2007 to May 2008 (182 patients) . | Narrow QRS blocks 84 P . | Wide QRS Blocks 98 P . |
|---|---|---|
| Block corrected | 82 (97.6%) | 51 (52%), P < 0.01 |
| Block not corrected | 0 | 39 (39.8%) |
| Not recorded, His not captured | 2 (2.4%) | 8 (8.2%) |
| Acute threshold for 1 ms | 2.1 ± 0.9 V | 2.6 ± 1.1 V, P < 0.05 |
| Rejected due to threshold >2.5 V | 17 (21%) | 25 (49%), P < 0.01 |
| Accepted for electrode implant | 65 | 26 |
| Successful fixation | 44 (67.7%) | 15 (57%) |
| ‘ R ’ amplitude | 8 ± 2.5 mV | 5 ± 1.8 mV, P < 0.01 |
| Success rate for total possible implants | 53% | 29%, P < 0.01 |
| Acute threshold in implanted patients (1 ms) | 1.4 ± 0.6 V | 1.9 ± 1.2 V, NS |
| Impedance | 334 ± 56 Ω | 328 ± 38 Ω, NS |
| Pattern of capture | Pattern I, 18 | Pattern A, 4 |
| Pattern II, 26 | Pattern B, 11 | |
| EF (%) | 57 ± 8* | 37 ± 4** |
| Follow-up (at 3 months) | ||
| Threshold for 0.5 ms | 1.9 ± 0.8 V, | 2.4 ± 1.4 V, NS |
| Impedance | 350 ± 22 Ω | 322 ± 43 Ω, NS |
| ‘ R ’ amplitude | 5.6 ± 1.8 mV | 4.4±1.1 mV, NS |
| Lost of capture/ dislodgement | 0/2 | 1/0 |
| EF (%) | 62 ± 6* | 42 ± 5** |
EF, ejection fraction. Values are means ±SD; (* P <0.01); (** P <0.01).
The His-bundle area was mapped with the lead (helix retracted), giving an average His-bundle threshold of 2.2 ± 1.1 V for 1 ms, clearly higher than that traditionally obtained for the right ventricular outflow tract or apex. A total of 42 patients (32% of potential candidates) were ruled out before the lead implantation was attempted because the His-bundle threshold was higher than 2.5 V ( Table 1 ). In addition, the His-bundle capture threshold was significantly higher in blocks with a wide QRS complex than those of narrow QRS ( Table 2 ).
No patient was rejected due to His-ventricular conduction block at rates lower than 120 b.p.m. Permanent fixation of the lead in the His region was achieved in 68% of attempts in the presence of blocks with a narrow QRS complex and in 57% of those with wide QRS complex ( Table 2 ). Successful implantation was achieved in 53% of all candidates who had a block with a narrow QRS complex, as compared with 29% for those with a wide QRS complex block ( Table 2 ).
Stable HBP was achieved in four of the eight patients in whom resynchronization with a built-in defibrillator was not possible via coronary sinus. The His-bundle lead withstood maximum power defibrillation without any dislodgement.
During follow-up, the pacing thresholds, impedances, and R-wave amplitude remained stable. Two leads were dislodged (both in patients who had a block with narrow QRS complex); loss of capture was observed in one patient in the wide QRS group. None of these patients had severe symptoms; the first two because they had spontaneous rhythm and the third because an additional lead was implanted in the right ventricle. The LVEF improved significantly in both groups ( Table 2 ).
Similar results were obtained when we compared patients with pure His-bundle capture with patients in whom fused capture was obtained ( Table 3 ).
Left ventricle ejection fraction (LVEF) and QRS duration in patients with pure and fused His bundle pacing (HBP)
| . | Pure HBP (22 patients) . | Fused HBP (37 patients) . | ||
|---|---|---|---|---|
| . | Before HBP . | 3 Months after HBP . | Before HBP . | 3 Months after HBP . |
| LVEF | 53% ± 7 | 55% ± 3 | 49% ± 5 | 53% ± 3 |
| P | 0.22 (NS) | <0.01 | ||
| QRS (ms) | 95 ± 6 | 85 ± 3 | 107 ± 4 | 102 ± 7 |
| P | <0.01 | <0.01 | ||
| . | Pure HBP (22 patients) . | Fused HBP (37 patients) . | ||
|---|---|---|---|---|
| . | Before HBP . | 3 Months after HBP . | Before HBP . | 3 Months after HBP . |
| LVEF | 53% ± 7 | 55% ± 3 | 49% ± 5 | 53% ± 3 |
| P | 0.22 (NS) | <0.01 | ||
| QRS (ms) | 95 ± 6 | 85 ± 3 | 107 ± 4 | 102 ± 7 |
| P | <0.01 | <0.01 | ||
Left ventricle ejection fraction (LVEF) and QRS duration in patients with pure and fused His bundle pacing (HBP)
| . | Pure HBP (22 patients) . | Fused HBP (37 patients) . | ||
|---|---|---|---|---|
| . | Before HBP . | 3 Months after HBP . | Before HBP . | 3 Months after HBP . |
| LVEF | 53% ± 7 | 55% ± 3 | 49% ± 5 | 53% ± 3 |
| P | 0.22 (NS) | <0.01 | ||
| QRS (ms) | 95 ± 6 | 85 ± 3 | 107 ± 4 | 102 ± 7 |
| P | <0.01 | <0.01 | ||
| . | Pure HBP (22 patients) . | Fused HBP (37 patients) . | ||
|---|---|---|---|---|
| . | Before HBP . | 3 Months after HBP . | Before HBP . | 3 Months after HBP . |
| LVEF | 53% ± 7 | 55% ± 3 | 49% ± 5 | 53% ± 3 |
| P | 0.22 (NS) | <0.01 | ||
| QRS (ms) | 95 ± 6 | 85 ± 3 | 107 ± 4 | 102 ± 7 |
| P | <0.01 | <0.01 | ||
No local or systemic infections were observed in the 91 patients in whom we attempted to implant the His-bundle lead.
His-pacing electrocardiogram patterns
Two patterns were found in the presence of AV blocks with a narrow QRS complex ( Tables 1 and 2 ):
Pure, unique His-bundle capture: The pure, direct, unique His-bundle capture shows latency between the pacing spike and the QRS complex which is identical to the native ( Figure 1 B ).
Fused His-bundle capture: The fused QRS complexes are of widened morphology, anteroseptal pre-excitation pattern, and show no latency with repolarization abnormalities ( Figure 1 B ).
Three main patterns are observed in the presence of AV block with a wide QRS complex ( Tables 1 and 2 ):
Presence of latency with the disappearance of bundle-branch and complete AV blocks and QRS normalization ( Figures 2 A , C , and 3 A ).
Absence of latency with the disappearance of bundle-branch blocks and complete AV blocks without QRS normalization ( Figure 3 A and C ).
Presence of latency and persistent branch blocks ( Figure 3 B and C ) or complete infra-Hisian AV block. These patients were considerable not suitable candidates for HBP.
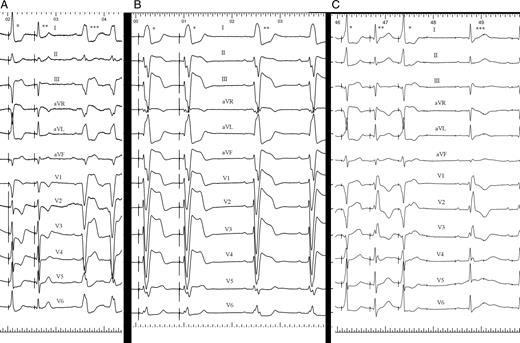
( A ) ECG showing sinus rhythm and left branch block (triple asterisks). A single asterisk marks the QRS resulting from the fusion of His-bundle capture and adjacent myocardial capture, double asterisks marks the QRS of pure His-bundle capture, which is the central left bundle-branch block. ( B ) ECG showing left bundle-branch block sinus rhythm (double asterisks). The QRS marked with a single asterisk is caused by His-bundle pacing with pure His-bundle capture and has the same morphology and duration as native QRS. This is a peripheral left branch block. ( C ) ECG showing sinus rhythm, first-degree AV block, and right bundle-branch block (triple asterisks). The QRS complex marked with a single asterisk is fused, making the right bundle-branch block disappear without QRS normalization. The QRS marked with double asterisks is a pure His-bundle capture, showing latency and native right bundle-branch block. This is a peripheral right bundle-branch block.
Discussion
In the present series, 73% of AV conduction disturbances which required permanent pacing, regardless of location, were corrected with HBP. However, this was achieved in only 64.8% of cases in which His-bundle lead implantation was attempted (44.3% of all potential patients). The results were more encouraging for narrow-QRS blocks, where success was achieved in 67.7% of cases attempted (53% of all potential cases). In patients who have a block with wide QRS complex, success was achieved in 57.6% of all attempts (29% of all potential patients).
In case of an AV block with a narrow QRS complex, pure His-bundle capture induces a QRS complex with a morphology and repolarization identical to native, because the pulse is conducted to the ventricles via a specific conduction system. The latency is equivalent to the conduction time from the His-bundle capture region to the start of ventricular depolarization and is also equivalent to the HV interval. Fused capture may be explained as capture of both the His bundle and the adjacent myocardium, giving rise to two activation fronts: one via a specific conduction system and the other via right anteroseptal myocardium. The QRS complexes are the result of the fusion of both fronts, which explains the absence of latency and the pre-excited aspect of the QRS complex. Pure and fused captures can often be observed in the same electrocardiogram tracing, depending on output energy and lead contact with the His-bundle area ( Figure 1 B , 3A , and C ). We believe that the site where fused captures are observed can be accepted as definitive, given that with ‘fused’ capture, the left ventricle will depolarize via the Purkinje system, preventing left intraventricular asynchrony. 11 Although not the main purpose of our study, LVEF significantly improved in both groups ( Table 3 ), but this should be investigated in future studies specifically designed with this aim.
In the case of blocks with a wide QRS complex, HBP is known to be capable of correcting bundle-branch blocks, 23–26 and obtain a normal QRS complex in the presence of complete AV block considered ‘infra-Hisian’. 19 The theory of the longitudinal dissociation of the His bundle 23–26 explains these phenomena. According to this theory, the fibres ascribed to the right and left branches are histologically differentiated and isolated inside the trunk. Injury to the trunk may damage these fibres, showing up in the ECG as a bundle-branch block or complete block. Stimulation of the portion distal to the injury normalizes the QRS complex.
Hence, complete branch blocks conventionally considered ‘infra-Hisian’ could be classified according to the site as either central (His bundle) or peripheral (branches or Purkinje system), depending on whether or not they disappear with HBP.
Pattern A would occur in the presence of a central block, when the His-bundle capture is ‘pure’ and distal to the blocked region; ventricular depolarization occurs via the Purkinje system, explaining the QRS normalization with latency.
Pattern B would be a ‘fusion’ caused by capture of the His bundle and the adjacent myocardium. Right ventricular pre-excitation would be present and would induce loss of latency and, in the presence of right bundle-branch block, would cause it to disappear independently of its central or peripheral site due to the right ventricular pre-excitation.
In the presence of left bundle-branch block (LBBB), Pattern B would be found solely at its central site, given that the QRS complexes would be a fusion between His-bundle and myocardial capture. The former would induce left ventricular activation via Purkinje, explaining the disappearance of LBBB. The latter pre-excites the right ventricle, which explains the loss of latency and absence of QRS normalization.
Pattern C is justifiable in the presence of a peripheral block with ‘pure’ HBP, explaining the persistent blocks with latency. This pattern can also be found in central blocks if His-bundle capture is pure and prior to the blocked region. All patients (48%) with this pattern were not considered eligible for HBP.
His-bundle pacing does entail greater energy consumption due to the higher stimulation threshold. A higher degree of fibrosis that causes a thicker layer of unexcitable tissue between lead and excitable myocardium, or calcification of this region, could explain this phenomenon. Patient selection for permanent HBP can be affected not only by this anatomic change, but also by the type of mapping. In our case, we preferred to map the His-bundle area with the permanent lead in order to avoid additional risk during the technique. However, if extensive mapping using the femoral approach has been performed, the rate of potential candidates for permanent HBP may be increased.
Lead placement in the His-bundle region can be difficult because it must be anchored parallel to the plane of contact with the AV septum, something for which it is not designed. The close proximity of the tricuspid valve and its movements contribute to the greater instability of the lead. At present, the technique may be tedious and time-consuming in some patients, because multiple attempts are necessary. This can increase the rate of complications (particularly, infection of the system), which is why we limited the time dedicated to the His-bundle lead implantation to no more than 20 min of fluoroscopy time and were in fact able to avoid infectious complications. The rate of successful implants could have been higher if we had not limited the procedure duration. In the future, however, new leads specifically designed for HBP with dedicated fixation systems should help reduce procedure duration, dislodgement rate, and fluoroscopy time. Nevertheless, the fact that the defibrillation threshold was tested with a maximum power shock in four patients, none of whom experienced His-bundle lead movement, and that dislodgement during follow-up was rare suggests that, the lead may remain in place in a high percentage of patients and the chance of dislodgement can be reasonably low.
Currently, in the presence of blocks with a wide QRS complex and because the His-region block can become enlarged and encompass the lead site, an additional safety lead must be inserted at the apex or right outflow tract to prevent asystolia, especially in patients with pure His-bundle capture. This lengthens the surgical procedure time and results in a higher cost. In the case of fused capture, it may be possible to avoid the safety lead because ventricular contraction is assured via myocardial capture.
We should emphasize that these are immediate and 3-month results; long-term results are not yet available for permanent HBP in infra-Hisian AV conduction disturbances related to thresholds and haemodynamic improvement.
Conclusions
In the present series, 44.3% of patients who required permanent cardiac pacing underwent HBP. Permanent HBP is easier to achieve in the presence of blocks with a narrow QRS complex than in those with a wide QRS complex, and HBP can be used to prevent left ventricular asynchrony or to facilitate recovery if it appears.
Loss of capture and dislodgement occurred in ∼5% of our patients, a level that was higher than in conventional right ventricular pacing; however, new lead designs should improve the pacing threshold and the fixation mechanisms. Furthermore, new types of batteries that are able to withstand higher energy consumption without significant shortening of their lifespan would be an advantage.
Conflict of interest: none declared.



