-
PDF
- Split View
-
Views
-
Cite
Cite
Kristina Hermann Haugaa, Ida Skrinde Leren, Knut Erik Berge, Jørn Bathen, Jan Pål Loennechen, Ole-Gunnar Anfinsen, Andreas Früh, Thor Edvardsen, Erik Kongsgård, Trond P. Leren, Jan P. Amlie, High prevalence of exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia mutation-positive family members diagnosed by cascade genetic screening, EP Europace, Volume 12, Issue 3, March 2010, Pages 417–423, https://doi.org/10.1093/europace/eup448
Close - Share Icon Share
Abstract
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited cardiac disease predisposing to life-threatening arrhythmias. We aimed to determine the prevalence of arrhythmias and efficacy of β-blocker treatment in mutation-positive family members diagnosed by cascade genetic screening.
Relatives of six unrelated CPVT patients were tested for the relevant mutation in the ryanodine receptor-2 gene. Mutation carriers underwent an exercise test at inclusion time and 3 months after the initiation of β-blocker therapy in the highest tolerable dose. The occurrence of ventricular premature beats, couplets, and non-sustained ventricular arrhythmias (nsVT) were recorded in addition to the heart rate at which they occurred. Thirty family members were mutation carriers and were followed for 22 (13–288) months. Previous undiagnosed CPVT-related symptoms were reported by eight subjects. Exercise test induced ventricular arrhythmias in 23 of the 30 mutation carriers. On β-blocker treatment, exercise-induced arrhythmias occurred at a lower heart rate (117 ± 17 vs. 135 ± 34 beats/min, P = 0.02) but at similar workload ( P = 0.78). β-Blocker treatment suppressed the occurrence of exercise-induced nsVT in three of the four patients, while less severe arrhythmias were unchanged. One patient died during follow-up.
Exercise test revealed a high prevalence of arrhythmias in CPVT mutation carriers diagnosed by cascade genetic screening. β-Blocker therapy appeared to suppress the most severe exercise-induced arrhythmias, while less severe arrhythmias occurred at a lower heart rate.
Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited disorder characterized by stress-induced ventricular tachycardia, which may degenerate into ventricular fibrillation and sudden cardiac death. 1 Catecholaminergic polymorphic ventricular tachycardia may manifest as syncope or cardiac arrest in children and young adults. 2–4 Without treatment, a mortality rate of 30% has been reported within the age of 30 in symptomatic patients. 4 Oral β-blockers are considered to be the treatment of choice and therapy should be initiated early. 5 Diagnosing CPVT patients may be challenging since resting ECG, echocardiography, signal averaging ECG, and electrophysiological studies frequently are completely normal. However, an exercise test may reveal frequent ventricular premature beats (VPBs) or a ventricular tachycardia of characteristic bidirectional morphology. 5 Family histories often reveal previous cases of sudden death or unexplained syncopes. 6 The diagnosis may be verified by molecular genetic analysis.
Catecholaminergic polymorphic ventricular tachycardia is most commonly autosomal dominantly inherited and is caused by mutations in the ryanodine receptor-2 (RyR2) gene. 7 However, a rare autosomal recessive form caused by mutations in the calsequestrin-2 gene has also been identified. 8 The prevalence of CPVT has been estimated to be 1:10 000. 9
Family members of mutation-positive CPVT patients may be offered genetic screening, and mutation carriers may be treated with prophylactic β-blocker therapy. However, little is known about the prevalence of symptoms and electrocardiographic findings in otherwise healthy family members diagnosed by cascade genetic screening. It remains to be shown whether exercise-induced VPBs predict the risk of malignant arrhythmias and sudden death in CPVT patients. 10 Moreover, there is no consensus as to whether asymptomatic mutation carriers without stress-induced arrhythmias should be treated with β-blockers. Finally, there are sparse data as to which β-blocker should be the treatment of choice, 11 and varying effects of β-blocker therapy have been reported. 2 , 3 , 6 , 10 , 12 , 13
The purpose of this study was to determine the prevalence of arrhythmias and efficacy of β-blocker treatment in CPVT mutation-positive family members diagnosed by cascade genetic screening.
Methods
Study population
A total of 128 index patients were molecular genetic screened for RyR2 mutations as part of ordinary health care in patients with a phenotype compatible with CPVT or long QT syndrome. Genetic screening was performed as previously described. 14 Six unrelated index subjects with a clinical diagnosis of CPVT were found to be heterozygous for a mutation in the RyR2 gene. Two patients were heterozygous for mutation G2337V in exon 46, two were heterozygous for G4671V in exon 97, one for A2387V in exon 47 and one for R176Q in exon 8. Cascade genetic screening for the relevant mutation was performed in the six families and mutation carriers were included in the study. All participants underwent cardiological clinical examination, standard 12-lead ECG, and exercise test at each visit. Exercise test was repeated for minimum 3 months after initiation of the highest tolerable β-blocker dose.
Echocardiographic examination was performed at inclusion with particular focus on the morphology and function of the right ventricle regarding the differential diagnosis of arrhythmogenic right ventricular cardiomyopathy.
Signal averaging ECG was performed and analysed as described earlier. 15 , 16
The study complies with the Declaration of Helsinki. Written informed consent was given by all study participants. The study was approved by the Regional Committee for Medical Research Ethics.
Exercise test
Bicycle exercise tests were performed starting at a workload of 50 W, which was increased by 25 W every second minute until exhaustion. Twelve-lead ECG was obtained at the start, monitored continuously, and recorded throughout the examination if ventricular arrhythmias were observed. Maximum heart rate and blood pressure were recorded as well as the heart rate and the workload for the appearance of arrhythmias. In addition, the most severe arrhythmia during exercise test and heart rate at which it appeared was recorded. Exercise test was performed as treadmill test and the use of a modified Bruce Ramp protocol 17 in four children below 13 years of age.
The arrhythmias were categorized into three classes: (i) frequent single VPBs, including bigeminy; (ii) VPB couplets; and (iii) non-sustained ventricular tachycardia (nsVT), including bi-directional ventricular tachycardia.
Statistical analyses
Continuous variables are presented as mean ± SD or median (range). Student's paired two-sided t -test was used for comparisons of continuous variables and Fisher's exact test was used for categorical variables before and after β-blocker therapy. P -values below 0.05 were considered statistically significant.
Results
A total of 30 family members were mutation positive. Figure 1 and Table 1 summarize the findings and follow-up. Sixteen family members were heterozygous for mutation G2337V, six for G4671V, six for A2387V, and two were heterozygous for R176Q. In one of the affected families, family history revealed that eight family members had died suddenly at a median age of 16 (10–43) years. Three of these were autopsied with no cause of death being identified. Post-mortem genetic testing in these three subjects who died at an age of 10, 24, and 43 years, respectively, revealed that they were all heterozygous for mutation G2337V in the RyR2 gene.
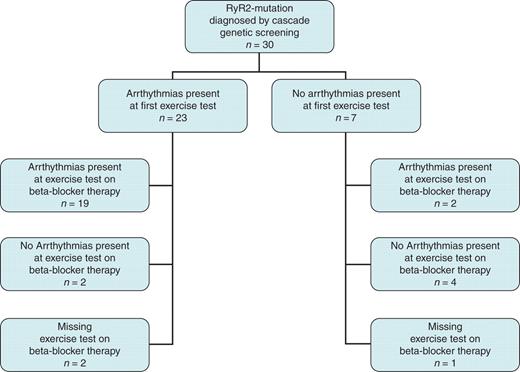
Study population overview. Overview of cascade genetic screening and findings by exercise test in 30 CPVT mutation carriers.
Clinical characteristics and β-blocker therapy in 30 CPVT mutation carriers diagnosed by cascade genetic screening
| Age (years) | 32 (6–75) |
| Females ( n ) | 16 (53%) |
| Follow-up (months) | 22 (13–288) |
| Heterozygous G2337V carriers ( n ) | 16 |
| Heterozygous G4671 carriers ( n ) | 6 |
| Heterozygous A2387V carriers ( n ) | 6 |
| Heterozygous R176Q carriers ( n ) | 2 |
| ICD ( n ) | 3 |
| β-Blocker therapy | |
| Metoprololsuccinate ( n = 17) (mg) | 125 ± 50 |
| Nadolol ( n = 10/ n = 7, children excluded) (mg) | 77 ± 34/90 ± 30 |
| Carvedilol ( n = 1) (mg) | 25 |
| Bisoprolol ( n = 1) (mg) | 10 |
| Propranolol ( n = 1) (mg) | 120 |
| Age (years) | 32 (6–75) |
| Females ( n ) | 16 (53%) |
| Follow-up (months) | 22 (13–288) |
| Heterozygous G2337V carriers ( n ) | 16 |
| Heterozygous G4671 carriers ( n ) | 6 |
| Heterozygous A2387V carriers ( n ) | 6 |
| Heterozygous R176Q carriers ( n ) | 2 |
| ICD ( n ) | 3 |
| β-Blocker therapy | |
| Metoprololsuccinate ( n = 17) (mg) | 125 ± 50 |
| Nadolol ( n = 10/ n = 7, children excluded) (mg) | 77 ± 34/90 ± 30 |
| Carvedilol ( n = 1) (mg) | 25 |
| Bisoprolol ( n = 1) (mg) | 10 |
| Propranolol ( n = 1) (mg) | 120 |
Median (range), mean ± SD.
Clinical characteristics and β-blocker therapy in 30 CPVT mutation carriers diagnosed by cascade genetic screening
| Age (years) | 32 (6–75) |
| Females ( n ) | 16 (53%) |
| Follow-up (months) | 22 (13–288) |
| Heterozygous G2337V carriers ( n ) | 16 |
| Heterozygous G4671 carriers ( n ) | 6 |
| Heterozygous A2387V carriers ( n ) | 6 |
| Heterozygous R176Q carriers ( n ) | 2 |
| ICD ( n ) | 3 |
| β-Blocker therapy | |
| Metoprololsuccinate ( n = 17) (mg) | 125 ± 50 |
| Nadolol ( n = 10/ n = 7, children excluded) (mg) | 77 ± 34/90 ± 30 |
| Carvedilol ( n = 1) (mg) | 25 |
| Bisoprolol ( n = 1) (mg) | 10 |
| Propranolol ( n = 1) (mg) | 120 |
| Age (years) | 32 (6–75) |
| Females ( n ) | 16 (53%) |
| Follow-up (months) | 22 (13–288) |
| Heterozygous G2337V carriers ( n ) | 16 |
| Heterozygous G4671 carriers ( n ) | 6 |
| Heterozygous A2387V carriers ( n ) | 6 |
| Heterozygous R176Q carriers ( n ) | 2 |
| ICD ( n ) | 3 |
| β-Blocker therapy | |
| Metoprololsuccinate ( n = 17) (mg) | 125 ± 50 |
| Nadolol ( n = 10/ n = 7, children excluded) (mg) | 77 ± 34/90 ± 30 |
| Carvedilol ( n = 1) (mg) | 25 |
| Bisoprolol ( n = 1) (mg) | 10 |
| Propranolol ( n = 1) (mg) | 120 |
Median (range), mean ± SD.
Median age at the first examination was 32 (6–75) years. Eight mutation carriers reported previous syncopes during physical exercise, which had occurred at a median age of 15 (10–34) years. Two of these had been misdiagnosed with epilepsy and treated with anti-epileptic medication. Three mutation carriers had experienced palpitations, while the remaining 19 mutation carriers reported no CPVT-related symptoms. All patients had normal resting ECG and normal signal averaging ECG. Resting heart rate was 70 ± 18 beats/min. None of the subjects had echocardiographic findings suggestive of arrhythmogenic right ventricular cardiomyopathy (ARVC), and all had normal left ventricular function.
Exercise test
Among the 30 mutation carriers, 23 developed various arrhythmias during exercise test. Non-sustained ventricular tachycardia was induced in four, VPB couplets were induced in five and frequent VPBs were induced in 14. Figure 2 shows typical exercise-induced VPBs in a study participant. None of the subjects experienced sustained ventricular tachycardia or required external cardioversion. One of the six subjects, in whom no arrhythmia was induced, had already been started on β-blocker therapy due to hypertension prior to the first exercise test. Maximum exercise heart rate was 173 ± 25 beats/min ( Table 2 ). VPBs started at a mean heart rate of 135 ± 34 beats/min and at a workload of 121 ± 43 W.
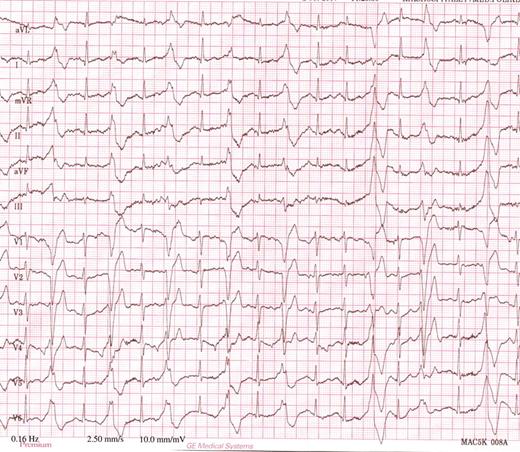
Typical ECG by exercise test in a heterozygous RyR2 mutation carrier. ECG shows multifocal ventricular premature beats with left bundle branch block configuration in bigeminy, at a heart rate of 120 beats/min.
Results from exercise test before and after the start of β-blocker therapy in 30 CPVT mutation carriers diagnosed by cascade genetic screening
| . | Before the start of bb-treatment . | After the start of bb-treatment . | P -value . |
|---|---|---|---|
| Heart rate at rest (b.p.m.) | 70 ± 18 | 54 ± 13 | <0.001 |
| Maximum heart rate during exercise test (b.p.m.) | 173 ± 25 | 139 ± 22 | <0.001 |
| Heart rate for debut of VPB (b.p.m.) | 135 ± 34 | 117 ± 17 | 0.02 |
| Workload for debut of VPB (W) | 121 ± 43 | 123 ± 43 | 0.78 |
| Heart rate at most severe arrhythmia (b.p.m) | 146 ± 30 | 124 ± 21 | <0.01 |
| Non-sustained VT | 4 | 1 | 0.14* |
| . | Before the start of bb-treatment . | After the start of bb-treatment . | P -value . |
|---|---|---|---|
| Heart rate at rest (b.p.m.) | 70 ± 18 | 54 ± 13 | <0.001 |
| Maximum heart rate during exercise test (b.p.m.) | 173 ± 25 | 139 ± 22 | <0.001 |
| Heart rate for debut of VPB (b.p.m.) | 135 ± 34 | 117 ± 17 | 0.02 |
| Workload for debut of VPB (W) | 121 ± 43 | 123 ± 43 | 0.78 |
| Heart rate at most severe arrhythmia (b.p.m) | 146 ± 30 | 124 ± 21 | <0.01 |
| Non-sustained VT | 4 | 1 | 0.14* |
b.p.m., beats per minute; VPB, ventricular premature beat; bb, β-blocker.
Mean ± standard deviation. P -value from paired Student's t -test and *Fisher's exact test.
Results from exercise test before and after the start of β-blocker therapy in 30 CPVT mutation carriers diagnosed by cascade genetic screening
| . | Before the start of bb-treatment . | After the start of bb-treatment . | P -value . |
|---|---|---|---|
| Heart rate at rest (b.p.m.) | 70 ± 18 | 54 ± 13 | <0.001 |
| Maximum heart rate during exercise test (b.p.m.) | 173 ± 25 | 139 ± 22 | <0.001 |
| Heart rate for debut of VPB (b.p.m.) | 135 ± 34 | 117 ± 17 | 0.02 |
| Workload for debut of VPB (W) | 121 ± 43 | 123 ± 43 | 0.78 |
| Heart rate at most severe arrhythmia (b.p.m) | 146 ± 30 | 124 ± 21 | <0.01 |
| Non-sustained VT | 4 | 1 | 0.14* |
| . | Before the start of bb-treatment . | After the start of bb-treatment . | P -value . |
|---|---|---|---|
| Heart rate at rest (b.p.m.) | 70 ± 18 | 54 ± 13 | <0.001 |
| Maximum heart rate during exercise test (b.p.m.) | 173 ± 25 | 139 ± 22 | <0.001 |
| Heart rate for debut of VPB (b.p.m.) | 135 ± 34 | 117 ± 17 | 0.02 |
| Workload for debut of VPB (W) | 121 ± 43 | 123 ± 43 | 0.78 |
| Heart rate at most severe arrhythmia (b.p.m) | 146 ± 30 | 124 ± 21 | <0.01 |
| Non-sustained VT | 4 | 1 | 0.14* |
b.p.m., beats per minute; VPB, ventricular premature beat; bb, β-blocker.
Mean ± standard deviation. P -value from paired Student's t -test and *Fisher's exact test.
β-Blocker therapy and its effect on exercise-induced arrhythmias
All 30 mutation carriers were treated with maximum tolerated doses of β-blockers. Of these, 12 subjects were treated with non-selective β-blockers [nadolol 77 ± 34 mg ( n = 10), carvedilol 25 mg ( n = 1), and propranolol 120 mg ( n = 1)], whereas 18 were treated with β1-selective blockers [metoprololsuccinat 125 ± 50 mg ( n = 17) and bisoprolol 10 mg ( n = 1)] ( Table 1 ).
Of the 30 mutation carriers, 27 underwent a second exercise test after the start of β-blocker therapy during a median follow-up of 22 (13–288) months. Resting heart rate and maximum heart rate were significantly reduced by 23 and 20%, respectively ( P < 0.001) ( Table 2 ). Among the 27 subjects, 23 had had exercise-induced arrhythmias before β-blocker therapy was started. In two of these subjects, no arrhythmias were observed after β-blocker therapy. Thus, 19 of the 23 subjects had persisting exercise-induced arrhythmias on maximum tolerated doses of β-blockers. The most severe arrhythmias observed during the second exercise test were nsVT in one, VPB couplets in four, and frequent VPBs in 16. Importantly, β-blocker therapy suppressed the occurrence of nsVT in three of the four patients ( P = 0.14). The patient with nsVT on β-blocker therapy was using an insufficient dose of metoprololsuccinat (50 mg), which was increased to 200 mg/daily. In four mutation carriers, a more severe arrhythmia was observed after β-blocker therapy ( Table 3 ). VPBs and most severe arrhythmias on β-blocker therapy appeared at 13 and 15% lower heart rate, respectively ( P = 0.02 and P < 0.01, respectively), but at a similar workload as without β-blocker therapy ( Table 2 ).
Effect of β-blocker therapy on exercise-induced arrhythmias in 30 CPVT mutation carriers diagnosed by cascade genetic screening
| Effect of β-blocker therapy . | n (%) . | Description of β-blocker effect . | ||
|---|---|---|---|---|
| . | . | n . | Before β-blocker . | After the start of β-blocker . |
| Arrhythmia suppressed | 2 (7) | 1 | Couplets | None |
| 1 | VPBs | None | ||
| Less severe arrhythmia | 4 (13) | 2 | Couplets | VPBs |
| 2 | nsVT | VPBs | ||
| No effect | 13 (43) | 10 | VPBs | VPBs |
| 2 | Couplets | Couplets | ||
| 1 | nsVT | nsVT | ||
| More severe arrhythmia | 4 (13) | 2 | None | VBPs |
| 2 | VPBs | Couplets | ||
| No arrhythmia before therapy | 4 (13) | |||
| Information not available | 3 (10) | |||
| Effect of β-blocker therapy . | n (%) . | Description of β-blocker effect . | ||
|---|---|---|---|---|
| . | . | n . | Before β-blocker . | After the start of β-blocker . |
| Arrhythmia suppressed | 2 (7) | 1 | Couplets | None |
| 1 | VPBs | None | ||
| Less severe arrhythmia | 4 (13) | 2 | Couplets | VPBs |
| 2 | nsVT | VPBs | ||
| No effect | 13 (43) | 10 | VPBs | VPBs |
| 2 | Couplets | Couplets | ||
| 1 | nsVT | nsVT | ||
| More severe arrhythmia | 4 (13) | 2 | None | VBPs |
| 2 | VPBs | Couplets | ||
| No arrhythmia before therapy | 4 (13) | |||
| Information not available | 3 (10) | |||
VPB, ventricular premature beat; nsVT, non-sustained ventricular tachycardia.
Effect of β-blocker therapy on exercise-induced arrhythmias in 30 CPVT mutation carriers diagnosed by cascade genetic screening
| Effect of β-blocker therapy . | n (%) . | Description of β-blocker effect . | ||
|---|---|---|---|---|
| . | . | n . | Before β-blocker . | After the start of β-blocker . |
| Arrhythmia suppressed | 2 (7) | 1 | Couplets | None |
| 1 | VPBs | None | ||
| Less severe arrhythmia | 4 (13) | 2 | Couplets | VPBs |
| 2 | nsVT | VPBs | ||
| No effect | 13 (43) | 10 | VPBs | VPBs |
| 2 | Couplets | Couplets | ||
| 1 | nsVT | nsVT | ||
| More severe arrhythmia | 4 (13) | 2 | None | VBPs |
| 2 | VPBs | Couplets | ||
| No arrhythmia before therapy | 4 (13) | |||
| Information not available | 3 (10) | |||
| Effect of β-blocker therapy . | n (%) . | Description of β-blocker effect . | ||
|---|---|---|---|---|
| . | . | n . | Before β-blocker . | After the start of β-blocker . |
| Arrhythmia suppressed | 2 (7) | 1 | Couplets | None |
| 1 | VPBs | None | ||
| Less severe arrhythmia | 4 (13) | 2 | Couplets | VPBs |
| 2 | nsVT | VPBs | ||
| No effect | 13 (43) | 10 | VPBs | VPBs |
| 2 | Couplets | Couplets | ||
| 1 | nsVT | nsVT | ||
| More severe arrhythmia | 4 (13) | 2 | None | VBPs |
| 2 | VPBs | Couplets | ||
| No arrhythmia before therapy | 4 (13) | |||
| Information not available | 3 (10) | |||
VPB, ventricular premature beat; nsVT, non-sustained ventricular tachycardia.
The two subjects, in whom arrhythmias were no longer inducible after the start of β-blocker therapy, had been treated with the non-selective β-blockers nadolol or carvedilol, respectively. One 17-year old mutation carrier died during follow-up. He had no arrhythmias at first exercise test. He was treated with propranolol 120 mg/daily. Exercise test on this medication showed frequent VPBs and propranolol was increased to 180 mg/daily. He died suddenly a few days after the dose of propranolol had been increased.
Implantation of cardiac defibrillators
Three mutation carriers received an implantable cardioverter-defibrillator (ICD). The first subject was in need of a pacing device due to symptomatic sinus bradycardia caused by the β-blocker therapy and we chose to implant an ICD for tachy- as well as bradytherapy. No arrhythmias or inappropriate shocks were recorded during 31 months follow-up. The second mutation carrier who received ICD had experienced repetitive syncopes before any treatment starting at 10 years of age. No arrhythmias were found at the first exercise test, but a second exercise test on β-blocker therapy showed frequent VPBs. Medication compliance was not optimal and additionally he insisted on continuing competing sports activities. An ICD was therefore implanted as a result of individual clinical evaluation at 15 years of age. During 29 months follow-up, he had three inappropriate shocks due to sinus tachycardia; all occurred on days when β-blocker medication had not been taken due to poor compliance. No ventricular arrhythmias have been detected by the device. The third patient had syncopes from 13 years of age and was diagnosed by family screening due to her mother's sudden death. She was treated with nadolol and had no syncopes on this medication. However, the patient got anxious due to frequent VPBs despite treatment, and an ICD was implanted based on individual clinical evaluation when she was 30 years old. No arrhythmias requiring ICD therapy have been recorded by the device during 7 years of follow-up.
Discussion
This study showed a high prevalence of previously undiagnosed symptoms and exercise-induced arrhythmias in CPVT mutation carriers detected by cascade genetic screening. Including only mutation-positive family members and no index patients, our results emphasize the importance of identifying these individuals.
β-blocker therapy appeared to reduce the occurrence of exercise-induced nsVT. Less severe arrhythmias, however, persisted and occurred at a lower heart rate than before β-blocker therapy was started.
Prevalence of arrhythmias by exercise ECG and catecholaminergic polymorphic ventricular tachycardia penetrance
Exercise test induced ventricular arrhythmias in the majority (77%) of the mutation carriers in our study. Considering exercise-induced arrhythmias as a CPVT symptom, the 77% penetrance of CPVT in our study was comparable with the 71% penetrance observed by Postma et al . 3 Prevalence of exercise-induced arrhythmias in our study was relatively high compared with studies where both index patients and affected relatives were included (reported prevalence of 83% 6 and 65% 10 ).
Effect of β-blocker therapy on exercise-induced and spontaneous arrhythmias
The occurrence of nsVT was suppressed in three of the four patients by β-blocker therapy. The patient with nsVT, despite β-blocker therapy, received an insufficient dose of metoprololsuccinat, suggesting that most severe arrhythmias were reduced by β-blocker therapy. In contrast, less severe arrhythmias observed at exercise test were not reduced, despite maximum tolerated β-blocker doses ( Table 3 ). β-Blocker treatment suppressed exercise-induced arrhythmias completely in only two subjects (7%). Exercise-induced VPBs occurred at the same workload as prior to β-blocker therapy, but at a lower heart rate. Similarly, the most severe arrhythmia recorded occurred at a lower heart rate on β-blocker therapy ( P < 0.01). In our patients therefore, β-blocker treatment significantly reduced heart rate, but this did not protect against the adrenergic stimulus which induced the arrhythmias at a defined workload. This may reflect that the occurrence of exercise-induced arrhythmias in CPVT depends on the level of physical stress and sympathetic activation rather than on the heart rate per se . The protective mechanisms of β-blocker therapy in CPVT patients have to be further explored.
As expected, resting and maximum heart rates during the test were significantly reduced by β-blocker therapy. The relatively high maximum heart rate at exercise test, with and without β-blocker treatment, in our study population, may be due to the inclusion of children and adolescents.
In studies where both index patients and affected relatives were included, varying effects of β-blocker therapy on stress-induced arrhythmias have been observed. Bauce et al . 10 found that 65% of the subjects no longer had VPBs on β-blocker therapy, and that VPBs occurred at a higher heart rate than without β-blockers in the remaining subjects. Postma et al . 3 found that CPVT-related symptoms disappeared in 98% of the patients, but that some still had VPBs. Priori et al . 6 observed no ventricular tachycardia or ventricular fibrillation in 63% of the patients on β-blocker therapy. However, data on less severe arrhythmias were not presented.
One mutation carrier in our study died during follow-up. None of the remaining β-blocker treated mutation carriers experienced syncopes during follow-up. However, due to the relatively short follow-up caution should be exerted when interpreting whether the risk of spontaneous arrhythmias was reduced by the treatment.
Effect of different β-blockers
In our study, 12 mutation carriers received non-selective β-blockers (nadolol, propranolol, or carvedilol) and 18 received β1-selective β-blockers (metoprololsuccinat or bisoprolol). Exercise-induced arrhythmias were suppressed in two patients using non-selective β-blockers nadolol or carvedilol. Non-sustained ventricular tachycardia was suppressed in two patients using nadolol and in one using bisoprolol. One patient on propranolol died. This may reflect that there may be differences in the efficacy of β-blockers. In other studies, nadolol, metoprolol and propranolol, 3 , 6 or atenolol and acebutol 10 have been used. Leenhardt et al . 2 concluded that any β-blocker may be effective, but data of the relative efficacy of the different β-blockers on CPVT-related symptoms are sparse. However, in a recently published study by Hayashi et al . 11 , cardiac events were observed in 19% of subjects treated with nadolol and in 39% of subjects treated with other β-blockers during a mean follow-up of 7.9 years. β-Blocker treatment other than nadolol was an independent predictor for cardiac events, suggesting that nadolol in a sufficient dosage (>1.5 mg/kg) is a recommendable drug for CPVT patients.
The different β-blockers vary not only with respect to selectivity, but also with respect to lipid solubility, bioavailability, and half-life. Differences in patient inclusion criteria, patient compliance, and strategies for dosing β-blockers may also contribute to the differences reported for various β-blockers in patients with CPVT.
Preventive therapies in catecholaminergic polymorphic ventricular tachycardia mutation carriers
In our study, all mutation carriers received β-blocker therapy, including those without exercise-induced arrhythmias. Three recent clinical studies with ∼50 mutation carriers each have been conducted. 3 , 6 , 10 All these concluded that β-blocker therapy should be given to patients with arrhythmias. However, varying recommendations have been suggested regarding therapy for asymptomatic subjects without exercise-induced arrhythmias. Whereas Postma et al . 3 and Priori et al . 6 gave β-blockers only to those who had clinical symptoms or exercise-induced arrhythmias, Bauce et al . 10 gave β-blockers to all mutation carriers. Swan et al . 18 suggested that adult, asymptomatic mutation carriers would not need β-blocker therapy, whereas asymptomatic children and adolescents should be treated with β-blockers until the age of 20. The reason for discontinuing β-blockers at age 20 was the assumption of minimal risk of developing CPVT-related symptoms above this age, since the typical age of debut of CPVT is reported to be 7–9 years of age. 2 , 3 In contrast, Hayashi et al . 11 reported similar cardiac event rate among probands and affected relatives, and therefore recommended β-blocker treatment for all CPVT mutation carriers irrespective of the cardiologic findings. The importance of performing cascade genetic screening in families with CPVT was emphasized by these authors due to the high prevalence of events in the mutation-positive relatives. 11 Our data are in accordance with those of Hayashi et al ., showing high arrhythmia prevalence in mutation-positive family members. Two of the mutation carriers in our study had no arrhythmias at exercise test before the start of β-blocker therapy. One of these died during follow-up and the second received an ICD due to frequent VPBs later on. These findings suggest that β-blocker therapy should be given to all mutation-positive individuals, regardless of the presence of exercise-induced arrhythmias.
In one of the families in our study, eight members had previously died suddenly between age 10 and 43. Two of these died at an age of 31 and 43 years, respectively. These findings therefore suggest that treatment and preventive measures should be continued in adult mutation carriers.
Implantable cardioverter-defibrillator therapy in addition to β-blocker was chosen in three of our mutation carriers, who had all experienced syncopes before β-blocker treatment. Two of them had symptomatic VPBs despite β-blocker treatment. In one mutation carrier, β-blocker treatment induced symptomatic bradycardia requiring a pacing device. Implantable cardioverter-defibrillator therapy in CPVT patients has been debated, since neither efficacy nor safety of this therapy is fully clarified. Patients are often young individuals and inappropriate shocks are frequent. 19 Importantly, inappropriate shocks may trigger patient stress and thereby initiate a sequence of ventricular arrhythmias and finally arrhythmic storm and multiple shocks. On the other hand, even appropriate shocks may be inefficient. 20
A few therapeutic alternatives have been reported. Wilde et al . 19 showed effective arrhythmia control in CPVT patients performing left cardiac sympathetic denervation, which may be a valuable alternative especially for patients whose symptoms are not adequately controlled by β-blockade. Promising additional medical therapies with calcium channel blockers 12 and flecainide 21 have been reported in small number of patients.
Conclusion
We have shown that cascade genetic screening is an efficient tool for diagnosing relatives of index patients with CPVT. Our study showed that a substantial proportion of CPVT mutation-positive family members had previously undiagnosed CPVT-related symptoms and a high prevalence of exercise-induced arrhythmias. On β-blocker therapy, exercise-induced arrhythmias occurred at a lower heart rate compared with untreated. The occurrence of nsVT appeared to be reduced by β-blocker therapy, while less severe arrhythmias were not affected. Further studies are needed to determine the mechanisms of the protective effect of β-blockers and whether specific β-blockers are superior to others among patients with CPVT.
Conflict of interest: none declared.
Funding
This work was supported by the South-Eastern Norway Regional Health Authority.
References
Author notes
K.H.H. and I.S.L. contributed equally to this paper.



