-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Brambilla, Eraldo Occhetta, Martina Ronconi, Laura Plebani, Alessandro Carriero, Paolo Marino, Reducing operator radiation exposure during cardiac resynchronization therapy, EP Europace, Volume 12, Issue 12, December 2010, Pages 1769–1773, https://doi.org/10.1093/europace/euq356
Close - Share Icon Share
Abstract
To quantify the reduction in equivalent dose at operator's hand that can be achieved by placement of a radiation-absorbing drape (RADPAD) during long-lasting cardiac resynchronization therapy (CRT) procedures.
This is a prospective observational study that included 22 consecutive patients with drug-refractory heart failure who underwent implantation of a CRT device. The cases were randomly assigned to Group A (11 cases), performed without RADPAD, and to Group B (11 cases), performed using RADPAD. Dose equivalent at the examiner's hand was measured as Hp(0.07) and as a time-adjusted Hp(0.07) rate (mGy/min) with a direct reading dosimeter. The mean fluoroscopy time was 20.8 ± 7.7 min and the mean dose area product (DAP) was 118.6 ± 45.3 Gy cm2. No significant differences were found between body mass index, fluoroscopy time, and DAP between patients examined with or without RADPAD. The correlation between the fluoroscopy time and the DAP was high (R2=0.94, P < 0.001). Mean dose and dose rate measurement without the RADPAD at the finger and hand were Hp(0.07)=1.27 ± 0.47 mGy per procedure and Hp(0.07) rate = 0.057 ± 0.011 mGy/min, respectively. The dosage was reduced with the RADPAD to Hp(0.07) = 0.48 ± 0.20 (P < 0.05) and to Hp(0.07) rate =0.026 ± 0.008 (P < 0.001), respectively.
A mean reduction of 54% in the equivalent dose rate to the operator's hand can be achieved with the use of RADPAD. The use of the RADPAD in CRT devices implantation will make unlikely the necessity of limiting the yearly number of implants for high volume operators.
Introduction
Cardiac resynchronization therapy (CRT) is approved for the treatment of patients with New York Heart Association (NYHA) class III–IV symptoms, with ejection fractions ≤35%, and with QRS durations ≥120 ms on electrocardiograms.1 Several randomized studies have shown improvements in the functional class, quality of life, ejection fractions, and exercise capacity with CRT.2–6 Cardiac resynchronization therapy procedures are usually performed in the cardiac catheterization laboratory under fluoroscopic guidance. Due to the complexity of the procedure, implantation may be prolonged, resulting in considerable fluoroscopic exposure. Hence, electrophysiologists and support personnel may be exposed to considerable levels of radiation, depending on the laboratory workload and complexity of the procedures.7 The highest level of radiation exposure is usually expected at the hands of the implanting physicians, where maximum surface dose of 9.2 mSv in a single procedure and a mean surface dose of 1.2 mSv per procedure have been recently reported in the course of CRT implantation.8 The annual limit of the equivalent dose at operator' hand is 500 mSv according to International Commission on Radiological Protection (ICRP) recommendations.9 This would require severe restrictions to the maximum number of implantations per year performed by a single physician, in the worst case scenario. Use of a sterile, radiation-absorbing drape has been demonstrated to reduce occupational radiation exposure during femoral interventional radiology procedures,10 pace maker implantation,11 electrophysiology, and catheter ablations.12 The aim of this study was to quantify the degree of reduction in equivalent dose at operator's hand that can be achieved by placement of a radiation-absorbing drape, outside the beam path during long-lasting CRT procedures.
Methods
Inclusion criteria and study protocol
This is a prospective observational study that involved a single centre. The study population included 22 consecutive patients with drug-refractory heart failure who underwent implantation of a CRT device from February to July 2010. Eligibility criteria for CRT were: advanced heart failure (NYHA functional classes III–IV), depressed systolic left ventricular function (≤35%), and prolonged QRS duration (≥120 ms). A single cardiologist (E.O.) in one laboratory performed 22 consecutive pectoral device implantations (17 biventricular implantable cardioverter defibrillators and 5 biventricular pace makers) using pulsed fluoroscopy and anteroposterior and left anterior oblique views. The cases were randomly assigned to Group A (11 cases), performed without special shielding, and to Group B (11 cases), performed using commercially available disposable sterile bismuth and antimony-containing drapes (RADPAD Subclavian, Worldwide Innovation and Technologies, Overland park, KS, USA). The shielding properties are certified by the manufacturer as 75% at 90 kVp or 0.125 Pb equivalent. The rectangular 30 × 43 cm shields were supplied with a semi-lunar 9 cm wide window. Each shield was positioned onto the operative drape, with its long axis parallel to the patient's median line, so that it covered the surfaces inferior and lateral to the beam path. The semi-lunar window surrounded the implant folder. It was secured to the operative drape with a pre-applied adhesive strips. Care was taken to place the shield outside the fluoroscopic field of view to avoid feedback increases in the beam intensity (Figure 1).
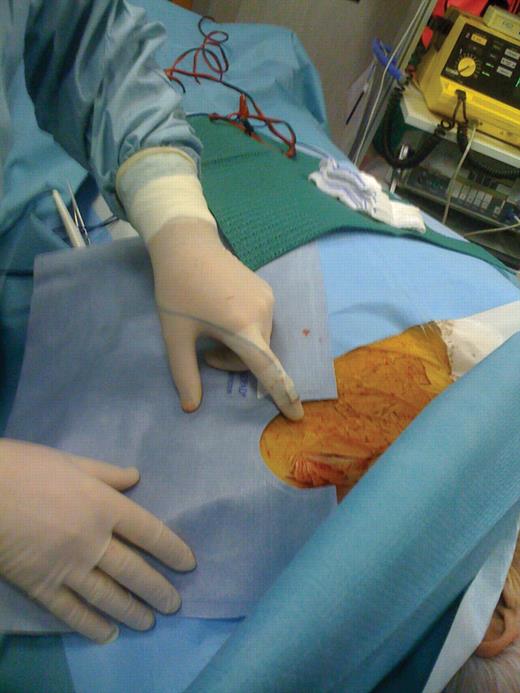
Positioning of the RADPAD during pectoral device implants. The shield is placed slightly lateral to the incision site and outside the beam path. Also shown in this figure is the positioning of the active dosimeter at operator's finger.
Device implantation
The left ventricle pacing lead was inserted by a transvenous approach through the coronary sinus, with an over-the-wire system, with a left subclavian access using standard operative techniques. The right atrium and right ventricle were stimulated by positioning standard bipolar leads in the right atrial appendage and right ventricular apex, respectively. Cardiac resynchronization therapy device and lead implantation were successful in all patients, without major complications.
Radiation exposure measurements
Dosimetric readings were obtained at the examiner's wrist (10 cases) and middle finger (12 cases) to monitor radiation exposure at the hand of the implanting physician. The dosimeter (Model NED, Educational Direct Dosimeter; Unfors Instruments, Billdal, Sweden) consists of a small sensor on a cable connected to a display unit. The NED is an instrument originally designed to measure finger doses during preparation and injection of radiopharmaceuticals. The calibration is performed at the radiation quality N-80 (80 kVp, 2 mm Cu, 4 mm Al) according to the ISO 4037 I standard. The Hp(0.07) for N-80 radiation quality is calculated from a conversion coefficient in ISO 4037-3 standard and determination of air kerma in free air, in absence of the phantom, at the point of phantom surface. The reading of the instrument, which are expressed as Hp(0.07) at 140 KeV photons, were referred to the N-80 radiation quality using the energy response curve provided by the manufacturer and the results of an inter-comparison of personal dose equivalent measurements by active personal dosimeters reported in a joint IAEA-EURADOS project.13 The measurement uncertainty of this dosimeter is ±6% (at the 95% confidence level). Hand doses to implanting physicians were also expressed as a time-adjusted Hp(0.07) rate (mGy/min) by dividing the measured Hp(0.07) by total fluoroscopy time in each procedure.
All studies were performed on a single-arm under-couch tube Siremobil intensifier system (Siemens Healthcare, Herlangen, Germany) with 40–110 kV and 0.2–8.9 mA operating range; the system was used with a pulsed fluoroscopy mode. In the majority of the cases, the intensifier field diameter was 23 cm. During the investigation, the fluoroscopy time (in min) and the dose area product [DAP (Gy cm2); type PTW Diamentor] were recorded. The tube voltage and the current dose were set automatically by an automatic exposure control device so that a patient entrance air kerma of 8.5 mGy/min is delivered when using a 20 cm PPMA phantom to simulate the patient. Under-couch table-attached 0.5 mm lead protection was used to shield from radiation the lower extremities of the operators. The operators used personal radiation protection devices such as lead glasses, collar, and an apron (lead equivalency 0.25 mm and 0.5 mm on the waist) in accordance with the ALARA principle recommended by the ICRP.
Statistical analysis
Baseline characteristics were compared between the 11 patients with RADPAD and the 11 patients without RADPAD using Mann–Whitney tests for non-normally distributed continuous variables.
Box and whiskers plots were used to provide a graphical representation of the variability of hand dose and dose rate to the implanting physician, assuming as a grouping variable the presence or the absence of RADPAD. Outliers and extremes are points higher than the value of the 75th percentile plus 1.5 or 3 times the inter-quartile distance, or lower than the value of the 25th percentile minus 1.5 or 3 times the inter-quartile distance, respectively. All data analysis was performed with Statistica 6.0 software (Statsoft) using a two-sided type I error rate of 0.05.
Results
Patient demographic data, fluoroscopy time, and DAP are shown in Table 1. The mean fluoroscopy time was 20.8 ± 7.7 min and the mean DAP was 118.6 ± 45.3 Gy cm which are in the same order (20.3 min and 111 Gy cm2) as those published by Butter et al.8 No significant differences were found between body mass index, fluoroscopy time, and DAP between patients examined with or without RADPAD. The correlation between the fluoroscopy time and the DAP (R2= 0.94, P < 0.001) is very high due to the absence of radiography mode during the different implantation tasks. Figure 2 shows the frequency distribution of DAP during CRT implantation procedures.
| . | RADPAD use . | P-value . | |
|---|---|---|---|
| . | No (n=11) . | Yes (n=11) . | . |
| Demographics | |||
| Age (year) | 76 ± 6 | 74 ± 4 | 0.16 (NS) |
| Men | 8 | 8 | 1.0 (NS) |
| BMI (kg/m2) | 28.1 ± 4.4 | 25.1 ± 4.0 | 0.53 (NS) |
| Exposure variables | |||
| Fluoroscopy time (min) | 22.9 ± 8.6 | 18.8 ± 6.5 | 0.45 (NS) |
| DAP (Gy cm2) | 127.6 ± 46.3 | 109.6 ± 44.6 | 0.49 (NS) |
| Dosimetric variables | |||
| Hp(0.07) (mGy) | 1.27 ± 0.47 | 0.48 ± 0.20 | 0.032 |
| Hp(0.07) rate (mGy/min) | 0.057 ± 0.011 | 0.026 ± 0.008 | 0.0009 |
| . | RADPAD use . | P-value . | |
|---|---|---|---|
| . | No (n=11) . | Yes (n=11) . | . |
| Demographics | |||
| Age (year) | 76 ± 6 | 74 ± 4 | 0.16 (NS) |
| Men | 8 | 8 | 1.0 (NS) |
| BMI (kg/m2) | 28.1 ± 4.4 | 25.1 ± 4.0 | 0.53 (NS) |
| Exposure variables | |||
| Fluoroscopy time (min) | 22.9 ± 8.6 | 18.8 ± 6.5 | 0.45 (NS) |
| DAP (Gy cm2) | 127.6 ± 46.3 | 109.6 ± 44.6 | 0.49 (NS) |
| Dosimetric variables | |||
| Hp(0.07) (mGy) | 1.27 ± 0.47 | 0.48 ± 0.20 | 0.032 |
| Hp(0.07) rate (mGy/min) | 0.057 ± 0.011 | 0.026 ± 0.008 | 0.0009 |
Data are presented as mean ± SD or as number (%). BMI, body mass index; DAP, dose area product; Hp(0.07), personal dose equivalent to the hand.
| . | RADPAD use . | P-value . | |
|---|---|---|---|
| . | No (n=11) . | Yes (n=11) . | . |
| Demographics | |||
| Age (year) | 76 ± 6 | 74 ± 4 | 0.16 (NS) |
| Men | 8 | 8 | 1.0 (NS) |
| BMI (kg/m2) | 28.1 ± 4.4 | 25.1 ± 4.0 | 0.53 (NS) |
| Exposure variables | |||
| Fluoroscopy time (min) | 22.9 ± 8.6 | 18.8 ± 6.5 | 0.45 (NS) |
| DAP (Gy cm2) | 127.6 ± 46.3 | 109.6 ± 44.6 | 0.49 (NS) |
| Dosimetric variables | |||
| Hp(0.07) (mGy) | 1.27 ± 0.47 | 0.48 ± 0.20 | 0.032 |
| Hp(0.07) rate (mGy/min) | 0.057 ± 0.011 | 0.026 ± 0.008 | 0.0009 |
| . | RADPAD use . | P-value . | |
|---|---|---|---|
| . | No (n=11) . | Yes (n=11) . | . |
| Demographics | |||
| Age (year) | 76 ± 6 | 74 ± 4 | 0.16 (NS) |
| Men | 8 | 8 | 1.0 (NS) |
| BMI (kg/m2) | 28.1 ± 4.4 | 25.1 ± 4.0 | 0.53 (NS) |
| Exposure variables | |||
| Fluoroscopy time (min) | 22.9 ± 8.6 | 18.8 ± 6.5 | 0.45 (NS) |
| DAP (Gy cm2) | 127.6 ± 46.3 | 109.6 ± 44.6 | 0.49 (NS) |
| Dosimetric variables | |||
| Hp(0.07) (mGy) | 1.27 ± 0.47 | 0.48 ± 0.20 | 0.032 |
| Hp(0.07) rate (mGy/min) | 0.057 ± 0.011 | 0.026 ± 0.008 | 0.0009 |
Data are presented as mean ± SD or as number (%). BMI, body mass index; DAP, dose area product; Hp(0.07), personal dose equivalent to the hand.
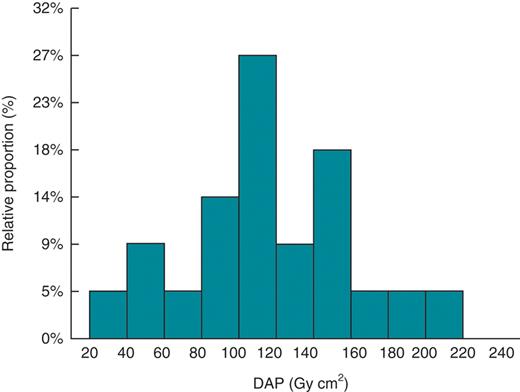
Frequency distribution of dose area product during cardiac resynchronization therapy implantation procedures.
Mean dose and dose rate measurements without the protective drape at the finger and hand were Hp(0.07) = 1.27 ± 0.47 mGy per procedure and Hp(0.07) rate = 0.057 ± 0.011 mGy/min, respectively. The dosage was reduced with the radiation protective device to Hp(0.07) = 0.48 ± 0.20 and to Hp(0.07) rate = 0.026 ± 0.008, respectively (Figure 3). This reduction was statistically significant and corresponded to a mean reduction of 54% in the measured dose rate. No significant differences were found between Hp(0.07) or time-adjusted Hp(0.07) rates between different positioning of NED dosimeter (finger or wrist) both without or with the use of RADPAD. A poor but significant correlation was found between DAP and dose at the operator's hand (R2= 0.40; P < 0.002).
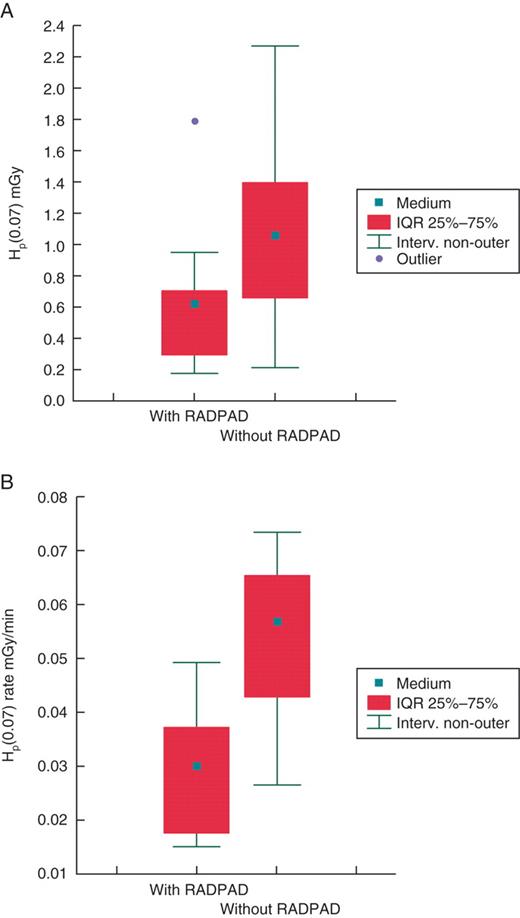
Box plot of showing the variability of skin dose of implanting physician expressed as Hp(0.07) (A) and time-adjusted Hp(0.07) rate (B) with and without the use of protective shields.
Discussion
In recent years, implantation of rhythm devices and cardioverter defibrillators has been proved to supersede the effectiveness of other therapeutic approaches and their application is increasing.14,15 Cardiac resynchronization therapy procedures often are associated with prolonged fluoroscopy times and, due to the complexity of the procedure, experienced operators often participate in multiple CRT implantations and revisions over an extended period of time. According to a recent study, dose at operator's hand may reach 9.2 mSv per procedure with a mean of 1.2 mSv per procedure.8 Due to the restriction regulated by national radiation directives16,17 that impose an annual limit of 500 mSv for the equivalent dose at operator's hand, the mean surface dose measured would require that the number of implantations per month be limited to 34 for the average case scenario. Among the protective options available in the electrophysiology room (including wraparound apron, thyroid shields, leaded glasses, under couch lead shielding), the leaded surgical gloves are the most controversial. These gloves are expensive due to their mono use prescription, provide limited attenuation of X-rays, decrease the tactile sensation and fine motor control in the fingers, and may lead the wearer to place his hands in the beam more often due to an increased sense of security. Kelsey and Mettler18 noted that gloves attenuation ranged from only 12 to 30% depending on the peak kilovoltage; this degree of protection could be erased if the operator's sense of security led to just 15 additional seconds of primary beam exposure during 5 min of a fluoroscopic procedure. Moreover, it has been demonstrated that forward-scattered and backscattered radiation contribute to hand exposure and that secondary electrons can penetrate in the most radiation sensitive layer of the hand skin, thus reducing the protective values of the gloves below the level demonstrated for narrow beam geometry.19 In our centre, surgical leaded gloves are neither prescribed by the radiation protection officer, nor used by interventional cardiologists or radiologists. On the other hand, the need for reducing the radiation detriment to laboratory staff is imperative and, in particular, the need for reducing hand doses to the implanting physicians. For instance, annual hand doses measured with thermo-luminescent dosimeter in a routine surveillance program to the implanting physicians (E.O.) underwent a steady rise since 2003 (12.6 mSv) to 2006 (45.9 mSv) to 2009 (74.0 mSv), reflecting the increased use of radiation in electrophysiology procedures. The routine use of RADPAD in pectoral implants should at least halve these doses.
The mean values of fluoroscopic times and DAP measured in this study are comparable to data reported in the literature for biventricular pacing device implantation procedures20 and for implantation and upgrade of CRT devices.8 In our study, a mean equivalent dose of 1.3 mSv per procedure without protective drape was measured at the hand of the implanting physician, which is well in agreement with the reported 1.2 mSv for a left access in similar procedures, while the maximum surface dose recorded was of 2.3 mSv against a reported maximum dose of 9.2 mSv.8 This discrepancy can be easily explained in consideration of the lower maximum DAP (204 Gy cm2) registered in our study when compared with a maximum DAP of 458 Gy cm2 reported by Butter et al.8 which, in turn, can be likely attributed to the different complexity of the individual procedures. Both the absence of significant differences in Hp(0.07) or time-adjusted Hp(0.07) rates between different positioning of NED dosimeter (finger or wrist) both without or with the use of RADPAD and the poor dependence of skin dose from DAP can be explained in the light of unforeseeable position of the hand during the procedure.
A significant mean reduction of 54% was achieved in the equivalent dose rate to the operator's hand with the use of a sterile drape, without beam-path interference despite the requisite angulated views and increased complexity of these case. This reduction must be seen also in the light of the practical impossibility of using ceiling-suspended lead shields (as those currently used during femoral-based procedures, such as coronary angiography or radiofrequency ablation) due to the surgical nature of the procedure and proximity of the operator to the incision site.
Study limitations
This is a single centre, observational study with a single experienced operator involved. The data may not necessarily generalize to other less-experienced centre and/or operators in absolute values as for mean fluoroscopy time, mean DAP, and mean hand equivalent dose or dose rates. Nevertheless, we are confident that the amount of percentage reduction in operator's hand exposure using the RADPAD protective drape generalizes since it is independent on operator's experience.
Only hand equivalent doses were assessed in the study, due to the availability of only one educational direct dosimeter. Again, we are confident that a similar percentage of reduction applies also to thyroid and to eye lens equivalent doses or to effective doses. We chose to monitor hand equivalent doses because they cannot usually be shielded by personal protective clothes and because they are more prone to exceed dose constraints, in this kind of procedures.
Finally, operators were not blinded with respect to the presence of RADPAD since no sham drape was used in procedures without RADPAD. It is unlikely that this circumstance will affect the conclusions of the present study. On the contrary, by first principles, the presence of protective drape should increase the sense of security of the operator and lead him to put his hands in the beam proximity more often. This in turn should increase the radiation rate measured in procedures with RADPAD.
Conclusions
The results of the present study demonstrated that a mean reduction of 54% in the equivalent dose rate to the operator's hand can be achieved with the use of a sterile drape, without beam-path interference despite the requisite angulated views and increased complexity of these case. The use of the sterile drape in CRT devices implantation will make unlikely the necessity of limiting the yearly number of implant for high volume operators.
Conflict of interest: none declared.



