-
PDF
- Split View
-
Views
-
Cite
Cite
Lorne J. Gula, David Massel, Damian P. Redfearn, Andrew D. Krahn, Raymond Yee, George J. Klein, Allan C. Skanes, Impact of routine transoesophageal echocardiography on safety, outcomes, and cost of pulmonary vein ablation: inferences drawn from a decision analysis model, EP Europace, Volume 12, Issue 11, November 2010, Pages 1550–1557, https://doi.org/10.1093/europace/euq306
Close - Share Icon Share
Abstract
The practice of routine vs. selective transoesophageal echocardiography (TEE) surveillance for left atrial appendage or intracavitary thrombus prior to pulmonary vein isolation (PVI) varies widely as evidence to guide this decision in terms of important clinical outcomes is lacking.
We constructed a decision analysis model to compare the cost-effectiveness of routine TEE for detection of left atrial thrombus vs. no TEE. The model incorporated health outcomes and costs. Markov methodology was used to follow patients as they transition through varying health states. We examined a hypothetical cohort of patients with symptomatic atrial fibrillation suitable for PVI, and expected outcomes were modelled over a period of 2 years. Simulated patients (SPs) undergoing a strategy of a routine TEE experienced significantly fewer transient ischemic attacks (TIAs) [OR 0.28 (0.22–0.37)], and debilitating strokes [OR 0.23 (0.15–0.33)]. Routine TEE led to an absolute risk reduction for stroke of 1.2% [number needed to treat (NNT) 84 (79–100)] and 1.9% for TIA [NNT 53 (48–59)]. The incremental cost-effectiveness ratio (ICER) for TEE was $226 608 per quality-adjusted life year (QALY). The ICER for TEE among high-risk SPs, with pre-existing clot in the left atrium, was $2232 per QALY.
Decision analysis and microsimulation suggest that routine use of TEE in an unselected population prior to PVI lowers the incidence of cerebral thrombo-embolic events but with considerable cost per QALY.
Introduction
Pulmonary vein isolation (PVI) is an effective therapy for the management of atrial fibrillation (AF).1–3 This procedure entails an atrial trans-septal approach, catheter manipulation within the left atrium and left atrial appendage, the creation of numerous left atrial ablation lesions which are potentially thrombogenic, conversion of atrial fibrillation to sinus rhythm, and a procedure duration of several hours with up to three catheters situated in the left atrium throughout. All of these factors give rise to concern regarding the potential for stroke as a result of either de novo thrombus formation in the left atrium or embolism of a pre-existing clot.
It has become widespread practice to perform PVI with full anticoagulation using intravenous heparin once trans-septal access is achieved, and at least short-term anticoagulation with coumadin after the ablation.4 However, the practice of pre-ablation left atrial surveillance for left atrial appendage or intracavitary thrombus using transoesophageal echocardiography (TEE) varies widely as evidence to guide this decision in terms of important clinical outcomes is lacking.5 Large-scale randomized trials are impractical given the low peri-procedural thrombo-embolic event rates and the compelling response to clot once detected on TEE. Given the paucity of evidence, we developed a decision analytic model to assess the efficacy and costs of pre-PVI TEE using a Markov process6 and Monte Carlo simulation.
Methods
We constructed a Markov decision analysis model (Figure 1) to compare the cost-effectiveness of two approaches to pre-PVI assessment: (ii) routine TEE for detection of left atrial thrombus (LAT); or (iiii) no TEE. Our model allowed us to incorporate both health outcomes and costs. The Markov methodology was used to allow the model the capacity to follow patients as they transition through varying health states. We examined a hypothetical cohort of patients with symptomatic atrial fibrillation deemed suitable for PVI. Expected outcomes were modelled over a period of 2 years subsequent to scheduled PVI. The decision tree and Markov model were created and analysed using TreeAge Pro 2006 v1.2 (TreeAge Software Inc., Williamstown, MA, USA).
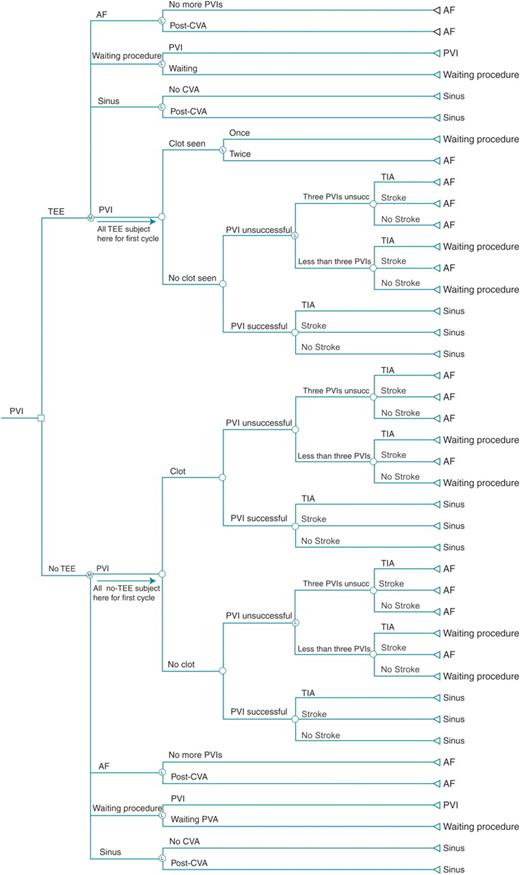
Decision model of transoesophageal echocardiography strategy prior to pulmonary vein isolation. The first node is a decision node regarding transoesophageal echocardiography or no transoesophageal echocardiography. The second nodes are Markov nodes, with all patients starting at the respective pulmonary vein isolation branch and then cycling through the model according to prespecified probabilities (Table 1). AF, atrial fibrillation; CVA, cerebrovascular accident; PVI, pulmonary vein isolation; TIA, transient ischemic attack.
Decision model
In the baseline model, patients enter the Markov cycle when the decision is made to perform a PVI. Each cycle constitutes a 1-month period. The primary outcome is quality-adjusted life months (QALMs) and secondary outcome is cost-effectiveness.
Surveillance strategies
In the routine TEE arm, all patients undergo a TEE prior to the initial PVI procedure and any subsequent PVI procedures. The probability of detecting an LAT was derived from an overview of published studies7–10 (Table 1). If no LAT is detected, the PVI procedure is carried out, with isolation of pulmonary vein conduction and intraoperative administration of intravenous heparin as per guidelines.11,12 If an LAT is detected, the patient is considered ‘high risk’ of thrombo-embolic complication of PVI, and the PVI procedure is deferred while the patient is transferred to the ‘waiting procedure’ state and anticoagulation with coumadin is continued. After 6 months, the patient undergoes a repeat TEE. In the no-TEE arm, patients undergo PVI, but it is assumed that patients with LAT are at a higher risk of TIA or cerebrovascular accident (CVA).
Procedural results
A successful PVI will eliminate symptoms related to atrial fibrillation. It is assumed that patients with recurrence of symptomatic AF after PVI (which is an unsuccessful procedure) will be offered, and will accept, a subsequent PVI procedure. A maximum of three PVIs will be performed for a given patient before AF is accepted as the permanent health state. Time between PVI procedures is 6 months to allow for a 3-month surveillance period and another potential 3-month booking time. Furthermore, it is assumed that a patient suffering a debilitating CVA as a result of the initial PVI will not undergo repeat PVI even if the first procedure is unsuccessful. Independent of PVI success, the patient is at risk for a peri-procedural TIA or CVA. We assume that stroke risk outside the peri-procedural timeframe is unchanged by PVI, and therefore coumadin management is guided by the CHADS2 score,13 with the exception that coumadin will be administered empirically for at least 2 months after PVI as per guidelines.11,12
Quality of life is determined by a composite of the quality-of-life score combining procedural outcome and presence or absence of complications. After PVI, the patient returns to the Markov node at the appropriate ‘health state’. If the PVI was successful, the patient is assigned to a ‘sinus’ state. Alternatively, the patient is assumed to be in a ‘waiting procedure’ state if the PVI was unsuccessful in the absence of complication, while a subsequent ablation is pending. Patients are assumed to be in a long-term ‘AF’ state if: (i) the PVI was unsuccessful and the patient experienced a CVA; (ii) two consecutive TEEs demonstrated presence of LAT; or (iii) there have been three failed attempts at PVI.
Although patients are at risk for complications unrelated to thrombo-embolic events and unpredictable by TEE (bleeding, cardiac perforation, pulmonary vein stenosis, etc.), only risks that may be differentially affected by TEE results are included in the model. This will result in a small absolute overestimate of quality-of-life measures and underestimate of costs, but will have no impact on relative measures between the TEE and no-TEE groups, which are the focus of this study.
Probabilities and rates
Estimates of base-case risk were derived from published data pertaining to patients with atrial fibrillation and thrombo-embolic sequelae of ablation procedures and cardioversion. Specific risks, their potential distribution range, and relevant citations are detailed in Table 1. The success rate has been selected to reflect the average reported success of PVI for patients with paroxysmal AF. The following variables deserve specific emphasis. The risk of an LAT detectable by TEE in a patient with AF was pessimistically assumed to be 4%. This is based on four recent studies of TEE findings during atrial fibrillation, described in Table 2. Risk of CVA is estimated at 20% if a clot is present and 0.3% if no clot is present.4,14
Quality-of-life estimates
All health states in our model were assigned a utility as a measure of effectiveness. All utilities are assigned a value from 0 to 1, with 0 defined as no quality of life (death) and 1 defined as full quality of life (perfect health). Utility of health states are based on published quality-of-life measures15–20 and are as follows: sinus rhythm after ablation 0.8 ± 0.05; atrial fibrillation 0.6 ± 0.03; TIA 0.79–0.90; CVA 0.1–0.7, with the latter two evenly distributed across the given range to account for variability of clinical effect. The use of QALMs allows incorporation into the model both the length of survival in a given health state and the quality of life during that survival. By definition, the maximum value for QALMs over the 2-year period of the model is 24.
It is assumed that patients who have undergone a successful PVI do not return to perfect health (utility of 1); the utility of patients in sinus rhythm following a PVI were converted from the Medical Outcomes Study Short Form 36 and based on published studies.19 Lastly, thrombo-embolic complications lead to a further decrement in quality of life and, specifically, are 0.85 for a TIA and 0.4 for a debilitating CVA.
Costs
All costs in the model are direct and take into account costs of all medical consequences of a given health event. Estimated costs are as follows in 2007 Canadian dollars: pulmonary vein ablation $8630; transoesophageal echocardiogram $585; TIA $3085/event;21 and CVA $9616/event + 1574/month.21 Costs of procedures reflect mean total costs at London Health Sciences Centre. Costs related to various complications are extrapolated from recent published data,21,22 and adjusted to 2007 dollar values according to the Health and Personal Care component of the Consumer Price Index. This analysis was from the perspective of the health-care system. Given the short-term duration of our model, neither costs nor utilities are discounted. It is assumed that a TIA will incur a head CT scan and an extension of hospital stay by 2 days. Costs of a CVA will include a head CT scan, a hospital stay of 2 weeks, and subsequent long-term care.
Sensitivity analyses
Quality-of-life measures and cost-effectiveness were calculated according to the initial decision on whether to perform TEE prior to PVI. To account for uncertainty in the probability, utility, and cost estimates, a four-step approach was used. First, a one-way sensitivity analysis was performed on all model variables. Secondly, two-way sensitivity analyses were conducted using the most sensitive variables. Thirdly, an analysis of extremes was performed by setting each variable individually, and in combination, to take the most pessimistic values from the point of view of quality of life, thereby generating a ‘worst-case’ scenario. Finally, a Monte Carlo simulation was performed with repeated sampling of each of the input variables' potential distribution ranges (Table 1), individually and in combination, calculating outcomes for each strategy. Outcomes from this ‘simulated clinical trial’ were examined as an illustration of actual outcomes that can be expected with each strategy.
Results
Base-case analysis
Number of procedures
Figure 2 summarizes the number of procedures, assuming a hypothetical cohort of 10 000 patients per treatment group. Among simulated patients (SPs) undergoing a routine TEE, 15 (0.15%) did not undergo any PVI owing to persistent LAT on repeated TEEs. Of the 69.5% of SPs who had only one PVI, 11 were a result of failed PVI but persistent LAT preventing future ablations, and 9 owing to CVA complicating the first ablation, thus preventing further procedures. With regard to the no-TEE strategy, 69.7% of SPs underwent only one procedure, including 29 due to a CVA complicating a failed procedure.
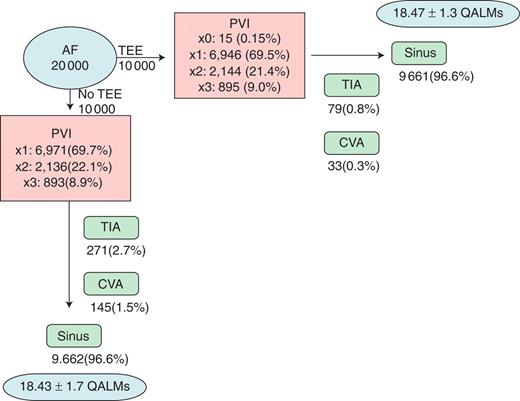
Monte Carlo results for all patients. Results are shown for Monte Carlo simulation of 10 000 patients in each strategy arm. Number of procedures, complications, outcomes, and cost-effectiveness are shown. AF, atrial fibrillation; CVA, cerebrovascular accident; PVI, pulmonary vein isolation; QALMs, quality-adjusted life months; TEE, transoesophageal echocardiogram; TIA, transient ischemic attack.
Outcomes
The probability of being in sinus rhythm 2 years after the decision to perform PVI is high (after multiple procedures), and does not differ between groups [96.61% with TEE and 96.62% no TEE, OR 1.0 (95% CI: 0.85–1.16), P = 0.97]. Simulated patients undergoing a strategy of a routine TEE have significantly fewer TIAs [OR 0.28 (95% CI: 0.22–0.37)], and debilitating strokes [OR 0.23 (95% CI: 0.15–0.33)].
A strategy of routine TEE led to an absolute risk reduction for stroke of 1.2% [number needed to treat (NNT) 84 (79–100)], and 1.9% for TIA [NNT 53 (48–59)]. Among the 145 SPs in the no-TEE strategy group with a CVA, 118 (81.3%) had a pre-existing clot in the left atrium and 27 (18.6%) did not. Outcomes are summarized in Figure 2.
Sensitivity analysis on the risk of CVA according to probability of clot and probability of event with or without clot present is summarized in Table 3. Varying probability of clot from 0 to 50% resulted in a 27.3% change in risk of TIA and 13.6% change in absolute risk of CVA.
One-way sensitivity analysis of factors affecting 2-year risk of cerebrovascular accident and transient ischemic attack
| Variable . | Range of values (%) . | Range of risk reduction with TEE (%) . |
|---|---|---|
| Probability of clot | 0–50 | 0–14 |
| Probability of CVA if no clot | 0.1–2.0 | 1.1–1.2 |
| Probability of CVA if clot | 1–50 | 0.1–2.8 |
| Probability of TIA if no clot | 0.1–5.0 | 2 |
| Probability of TIA if clot | 1–70 | 0–3.9 |
| Variable . | Range of values (%) . | Range of risk reduction with TEE (%) . |
|---|---|---|
| Probability of clot | 0–50 | 0–14 |
| Probability of CVA if no clot | 0.1–2.0 | 1.1–1.2 |
| Probability of CVA if clot | 1–50 | 0.1–2.8 |
| Probability of TIA if no clot | 0.1–5.0 | 2 |
| Probability of TIA if clot | 1–70 | 0–3.9 |
One-way sensitivity analysis of factors affecting 2-year risk of cerebrovascular accident and transient ischemic attack
| Variable . | Range of values (%) . | Range of risk reduction with TEE (%) . |
|---|---|---|
| Probability of clot | 0–50 | 0–14 |
| Probability of CVA if no clot | 0.1–2.0 | 1.1–1.2 |
| Probability of CVA if clot | 1–50 | 0.1–2.8 |
| Probability of TIA if no clot | 0.1–5.0 | 2 |
| Probability of TIA if clot | 1–70 | 0–3.9 |
| Variable . | Range of values (%) . | Range of risk reduction with TEE (%) . |
|---|---|---|
| Probability of clot | 0–50 | 0–14 |
| Probability of CVA if no clot | 0.1–2.0 | 1.1–1.2 |
| Probability of CVA if clot | 1–50 | 0.1–2.8 |
| Probability of TIA if no clot | 0.1–5.0 | 2 |
| Probability of TIA if clot | 1–70 | 0–3.9 |
Quality of life
The mean estimate of quality of life after 2 years was similar between groups of SPs. Out of a possible 24 QALMs, it was 18.47 ± 1.3 QALMs with TEE strategy and 18.43 ± 1.7 QALMs without. The range of QALMs was 5.76–19.2 in both strategies.
High-risk patients
Of the 10 000 hypothetical patients undergoing a strategy of routine TEE (Figure 3), it is expected that an LAT would be present in 545, and of these, it would have resolved in 530 (97.2%) after an additional 6 months of anticoagulation. These 530 SPs went on to PVI, with 481 (88.3%) eventually restored to sinus rhythm. In 545 SPs without TEE, 482 (88.4%) had successful restoration of sinus rhythm. The expected number of procedures required to achieve sinus rhythm in each arm is shown in Figure 3. Mean expected quality-of-life measure was 16.7 ± 1.4 QALMs over 24 months with TEE, and 15.9 ± 4.4 without.
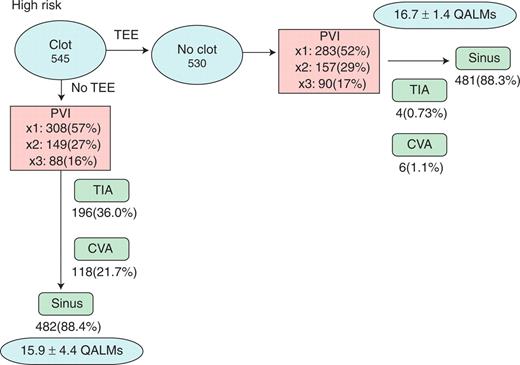
Monte Carlo results for high-risk patients. Results are shown for Monte Carlo simulation restricted to high-risk patients, as defined by the presence of pre-existing clot in the left atrium. AF, atrial fibrillation; CVA, cerebrovascular accident; PVI, pulmonary vein isolation; QALMs, quality-adjusted life months; TEE, transoesophageal echocardiogram; TIA, transient ischemic attack.
Among high-risk SPs, a strategy of routine TEE followed by deferral of the procedure and additional anticoagulation leads to a marked reduction in the risk of TIA and stroke. Among high-risk TEE SPs, there were 4 TIAs (0.73%) compared with 196 (36.0%) in patients without TEE (OR 0.013, P < 0.001). Cerebrovascular accidents occurred in 6 high-risk (1.1%) SPs with TEE, compared with 118 (21.7%) without TEE (OR 0.040, P < 0.001).
Low-risk patients
Among 9455 SPs without clot in the left atrium, 75 TIAs (0.79%) and 27 CVAs (0.29%) occurred in each arm. Successful ablation was ultimately achieved in 9180 (97.1%) in each arm. Quality of life was the same regardless of TEE among these patients, 18.6 ± 1.2 QALMs.
One-way sensitivity analyses
A series of one-way sensitivity analyses was performed. Expected quality of life was sensitive to change in four variables: utility of sinus rhythm, utility of atrial fibrillation, probability of PVI success, and initial probability of clot. The first two of these variables demonstrated threshold values which could alter preferential strategy. As utility of sinus rhythm was changed from 0 to 1, corresponding QALMs ranged from 2.4 to 22.8 with a threshold utility 0.6, above which ‘no TEE’ was the preferred strategy. As utility of atrial fibrillation was changed from 0 to 1, corresponding QALMs ranged from 16.8 to 20.0 with a threshold utility of 0.8, above which ‘TEE’ was the preferred strategy.
Costs and cost-effectiveness
Among 10 000 paired results of microsimulation, mean cost for SPs undergoing TEE was $13 500 ± 6971 (9215–27 645) (median 9215), and without TEE $12 715 ± 7524 (median 8630). The mean difference between the two groups was $785 ± 4188 and median difference $585. The incremental cost-effectiveness ratio (ICER) for TEE was $18 884 per additional QALM, or $226 608 per quality-adjusted life year (QALY).
Costs were assessed separately among the subset of high-risk SPs—those with clot in the left atrium at time of first planned PVI. Mean cost among these SPs with TEE strategy was $21 977 ± 6113 (median 18 430), and without TEE was $21 819 ± 17 280 (median 11 715). The ICER for TEE was $186 per QALM, and $2232 per QALY. The small difference between these costs is due to the long-term equilibration between cost of negative TEE and cost of stroke. The former occurs more frequently than the latter and, although less costly, the cumulative costs balance over time.
Discussion
A decision analysis approach to the question of TEE prior to PVI permits several clinically important calculations. In the absence of an enormous clinical trial, which would be necessary in light of low incidence rates, it combines the best available data on risks, benefits, and costs into a ‘simulated trial’. In contrast to a clinical trial, this type of simulated trial does not seek to determine success rates, complication rates, or costs, but combines estimates of these values from available evidence to assess what outcomes can be expected. This quantitative approach is useful as it is difficult to integrate the complex and comprehensive data across several trials purely by intuition, and then apply this to a system as complex as AF treatment which may entail several PVI procedures. Although best evidence-based estimates are used in decision analysis, and these values may not have consensus, the estimates are varied widely in the sensitivity analysis to assess whether change in any particular variable does, in fact, have an impact on outcomes and to what degree.
We have applied this method to the question of TEE prior to PVI, which has recently been the subject of much debate. Current guidelines recommend TEE in patients with persistent AF, and for those with significant atrial enlargement and risk factors for stroke.12 Although this traditionally pertains to a minority of patients, over 70% of ablation centres report a local requirement for TEE surveillance in all patients prior to PVA.4,23,24 Instrumentation of the left atrium, creation of electroanatomic maps that necessitate intimate contact with the entire endocardial surface, and delivery of ablation lesions which are potentially thrombogenic have compelled many to perform routine TEE before ablation regardless of clinical variables, and the infrequent discovery of a clot in these patients reinforces this practice. In contrast, some centres use clinical variables to assign risk levels to patients and perform TEE in a selected fashion. The evidence would suggest that TEE may be of benefit in this selected population, but cannot prevent embolic events entirely. Direct outcome evidence for routine TEE prior to PVI for atrial fibrillation is lacking, and must be extrapolated from studies of TEE in the context of external cardioversion of atrial fibrillation.9,10,25–27 Such studies have demonstrated that the use of TEE can reduce, but not exclude, the risk of cerebroembolic complications. Application of routine TEE has been associated with safe cardioversion in patients without prior anticoagulation, but studies have shown that TEE can demonstrate clot regardless of the prior anticoagulation state, in association with clinical variables such as LA size and LV function.9
A number of factors cause concern about thrombo-embolic risk at time of ablation for atrial fibrillation. This risk can be due to pre-existing left atrial thrombus, or de novo thrombus formation during or after the procedure. In an attempt to prevent pre-operative formation of thrombus, which can dislodge and embolize owing to catheter trauma, it has become nearly universal practice to prescribe coumadin prior to, and following, PVI.23,24 With regard to de novo thrombus formation, intravenous heparin is commonly used during PVI,23,24 and aggressive intraoperative anticoagulation (activated clotting time >300 s) has been shown to significantly reduce risk among patients with spontaneous echo contrast in the left atrium.28 Both of these anticoagulation strategies are supported by current guidelines.11,12 In spite of best efforts to prevent these complications, screening with magnetic resonance imaging after PVI has revealed silent cerebral emboli in up to 10% of patients without pre-procedural evidence of thrombus.29 Clinical thrombo-embolic events occur in a small percentage of patients, usually within 24 h of ablation.14
Use of TEE is associated with a low risk of side effects and the result is compelling when clot is identified. TEE, however, can be expensive and potentially adds significant time and discomfort to the patient's experience. Randomized trials would require numerous patients and would incur ethical debates if TEE results were blinded, despite the lack of relevant evidence. They would also require an enormous sample size, given the infrequent detection of atrial clot. Thus, we elected to construct a model assuming routine use of TEE vs. none. We estimated risks based on current data regarding incidence of clot and cerebral embolism; however, it is worth bearing in mind that a majority of these patients would have been considered low risk, with normal left ventricle, mildly dilated left atrium, frequent hypertension, no history of stroke, and age <70. Most patients undergoing PVI would therefore have a CHADS2 score13 of 1 for hypertension. Recent evidence indicates that CHADS2 score does correlate with TEE-detected clot and echo contrast.30 In this study, patients with CHADS2 score of 2 or less had a risk of clot or contrast of 5% or less, with an 11% risk in patients with the highest score of 6. This is consistent with our assumption that most PVI patients are relatively free of significant comorbidities.24 Useful risk factors that predict clot in the left atrium, assuming they exist, would need to extend beyond the conventional ‘CHADS2’ variables. Our model showed that the NNT to prevent TIA is 53 in an unselected population. Should the population considered for ablation expand to include older, more fragile patients, the incidence of clot will likely increase and the NNT might be lower in this selected group.
We believe that there is a subgroup of PVI patients at sufficiently low risk that TEE is not warranted. The results of our model lend quantitative support to this notion. We do acknowledge, however, that stroke is a disastrous complication and its low overall incidence will not be a comfort to those in whom it does occur. The routine TEE decision is therefore a struggle as it pits cost-effectiveness against the rare but compelling disastrous complication. In terms of ‘real-world’ clinical medicine, it is important to consider that management decisions may be poorly correlated to cost-benefit evidence when rare but disastrous complications are a factor. In such decisions involving uncertainty, classic expected utility theory suggests that the utility of the various outcomes should be weighted by the probability of their occurrence, and the preferred course of action the one which has the highest expected utility.31 While many factors can cause medical decision makers to deviate from expected utility theory, an important one is the anticipation of regret. This can play a role when a decision outcome is compared with an alternative outcome had a different decision been made. When anticipated regret is sufficiently high, evidence suggests that a decision maker will often choose to violate expected utility theory.32,33 There is also evidence to suggest that such decisions more often lead to action than omission.34 As such, the anticipated regret associated with the decision to forego TEE to rule out a thrombus, which then leads to a debilitating peri-procedural stroke, may be sufficient to favour a TEE even if the probability of a thrombus is extremely low.
Limitations
Our study is susceptible to the usual issues pertaining to decision and Markov models. The point estimates may not properly characterize the experience of all operators. To address this, the sensitivity analysis varies all estimates over a wide range and demonstrates that outcomes are very robust. The assumption that each ablation has a 30% failure rate results in a success rate over 90% after three procedures. This may be viewed as high by some. Evidence is consistent with this estimate, with success approaching 90% after two ablations.35 We also note that remaining atrial arrhythmias after two PVI procedures tend to be left atrial flutters, which are highly amenable to catheter ablation.
We acknowledge that there may be other benefits to TEE performed immediately prior to ablation, in that the TEE could then be used to guide the trans-septal puncture. This may impact on safety by reducing risk of perforation. As our analysis pertains to thrombo-embolic complications, we have not included this as a separate factor but rather incorporated overall risk of perforation into the risk estimate for the procedure.
Conclusions
Decision analysis and microsimulation suggest that routine use of TEE in an unselected population prior to ablation for atrial fibrillation lowers the incidence of cerebral thrombo-embolic events but with considerable cost per QALY. Further study is required to elicit factors that predict atrial thrombus to allow a more directed, cost-effective approach to pre-PVI TEE.
Conflict of interest: none declared.



