-
PDF
- Split View
-
Views
-
Cite
Cite
Yu-Feng Hu, Ching-Tai Tai, Yenn-Jiang Lin, Shih-Lin Chang, Li-Wei Lo, Wanwarang Wongcharoen, Ameya R. Udyavar, Ta-Chuan Tuan, Shih-Ann Chen, The change in the fluoroscopy-guided transseptal puncture site and difficult punctures in catheter ablation of recurrent atrial fibrillation, EP Europace, Volume 10, Issue 3, March 2008, Pages 276–279, https://doi.org/10.1093/europace/eun013
Close - Share Icon Share
Abstract
A second procedure for recurrent atrial fibrillation (AF) may be associated with the need for a different positioning of the puncture site and may increase the difficulty and complications. This study investigated whether the transseptal puncture site changed and whether the difficult punctures increased in the patients who received a repeat ablation procedure for recurrent AF.
Twenty-nine AF patients (52 ± 12 years old, 20 males) underwent catheter ablation for a recurrence of AF. Compared with the first procedure, the height between the transseptal puncture site and coronary sinus ostium was higher in the second procedure during both the atrial end-systolic phase (38.0 ± 4.7 vs. 34.8 ± 5.3 mm, P = 0.036), and end-diastolic phase (43.0 ± 4.8 vs. 39.1 ± 5.4 mm, P = 0.004) in the 30° right anterior oblique view. No significant change in the vertical atrial diameter was noted between the first and second procedures. A higher incidence of a difficult puncture was noted during the second procedure than in the first procedure (28 vs. 7%, P = 0.014). All those difficult punctures were overcome by using a large-curved transseptal needle. No differences of age, gender, AF duration, interval between first and second procedures, procedure time of the first procedure, and left atrial anteroposterior diameter were noted between easy and difficult transseptal punctures during the second procedure.
The incidence of a difficult puncture was higher in the second procedure compared with the first procedure. The transseptal puncture site moved higher in the second procedure. Chronic scarring over the previous transseptal site is a reasonable hypothesis to explain the observations. The difficult punctures experienced during the second procedure might be overcome by changing the needle curve from a small curve to a large curve design.
Introduction
The transseptal puncture technique can result in life-threatening complications. 1–3 In the study involving a worldwide survey on human atrial fibrillation (AF), 4 24.3% of the patients required a second procedure. Marcus et al . 5 reported 16 patients undergoing a repeat transseptal catheterization. Compared with the first procedure, the repeat transseptal catheterization after the ablation for AF was more difficult and potentially associated with more complications. Several studies 6 , 7 had demonstrated that atrial structural remodelling occurs after AF, which might change the atrial anatomy and transseptal puncture site for the repeat ablation. The transseptal puncture site was reported to move higher and more posterior with age. 8
The second procedure for recurrent AF may be associated with a need for a different positioning of the puncture site, an increase in the difficulty, and potentially increased complications. This important issue has not been extensively reported.
The purposes of this study were to measure the distances between the transseptal puncture sites and anatomical landmarks and to investigate the difference between the first and second ablation procedures.
Methods
Study population
From September 2003 to July 2006, 29 AF patients underwent the same transseptal and ablation technique for the first AF and recurrent AF (231 ± 195 days after the first procedure). The interval ranged from 43 to 876 days. There were 20 men and 9 women (mean age 52.5 ± 12 years, range 27–73 years). Eleven patients were associated with other cardiovascular diseases (four with hypertension, one with a coronary artery fistula, one with diabetes mellitus, and five with hyperlipidaemia).
Transseptal procedure and electrophysiological study
All patients provided informed consent for the procedure. Transthoracic echocardiography and transoesophageal echocardiography were performed before procedures. Pulmonary angiography in the venous phase was performed before the transseptal procedure in the 30° right anterior oblique (RAO) and 60° left anterior oblique (LAO) views. Two injections with ionized or non-ionized contrast medium were performed from the right and left pulmonary arteries under the rate of 10 cc/s. The standard elctrophysiological techniques were used as described in the authors’ previous work. 9–13 Through a femoral arterial access, a pigtail catheter was positioned just superior to the aortic valve. The transseptal sheath and dilator were advanced into the superior vena cava. With the Brockenbrough needle at the tip of the sheath, the sheath was torqued towards the atrial septum. Under fluoroscopic guidance (60° LAO), the sheath was pulled inferiorly to watch for the dilator jump under the aortic knob, and then a second jump under the muscular atrial septum onto the fossa ovalis. Two preferred methods to confirm the transseptal puncture site were to apply force with a palpable pop onto the fossa and to inject contrast dye in order to stain the septum. Once in the proper position, the Brockenbrough needle was advanced. Contrast was injected to assess the position of the Brockenbrough needle before advancing the transseptal dilator. In our laboratory, two different curved transseptal needles from the St Jude Medical company were used. One was a BRK (small-curved, the angle between the distal curved portion and shaft was ∼19°), and the other was a BRK1 (large-curved, the angle between the distal curved portion and shaft was ∼53°). Initially, the small-curved needle was routinely used. If the transseptal puncture failed after three attempts, the large-curved needle was used instead of the small-curved needle, and the procedure was repeated as described earlier. Having to change from the small-curved needle to the large-curved needle was defined as a difficult puncture.
Catheter ablation
Isolation of the four pulmonary veins (PVs) was performed from the atrial side of the PV antrum using the electrogram-guided approach (entrance block) simultaneous with the creation of the PV-left atrial geometry using a NavX system. The disappearance of all PV potentials in the PV antrum was confirmed by circular catheter recordings.
Definition and measurement of anatomical distances
The seating point of the electrode catheter in the coronary sinus was very close to the coronary sinus ostium (CSO), and that seating point of the catheter was considered as the zero point and was substituted for the CSO. All parameters were measured by electronic callipers on the digital images offline during the end-atrial diastolic and end-systolic phases as illustrated in Figure 1 . In the 30° RAO view, the definition of each parameter was (i) V N-CSO: the vertical distance between the transseptal puncture site and CSO; (ii) H N-CSO: the horizontal distance between the transseptal puncture site and CSO; (iii) V RS-CSO: the vertical distance between the right superior PV–left atrial junction and CSO; (iv) H RS-CSO: the horizontal distance between the right superior PV–left atrial junction and CSO; (v) V RI-CSO: the vertical distance between the right inferior PV–left atrial junction and CSO; (vi) H RI-CSO: the horizontal distance between the right inferior PV–left atrial junction and CSO; and (vii) VLA: the longest vertical distance between the roof and bottom of the left atrium. The PV–left atrial junction was defined as the point of inflection between PV wall and LA wall. In the 60° LAO view, the angle of the direction of the transseptal needle ( N -angle) was measured as the angle between the direction of the needle at the puncture site and horizontal line.
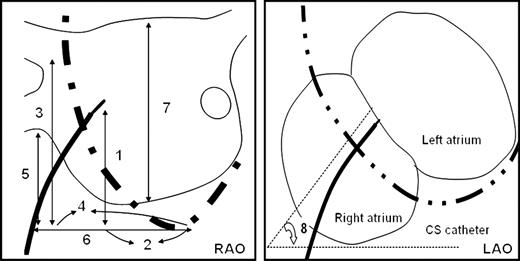
The measurements of the transseptal puncture site and adjacent structures. The lines with double arrows illustrate the various measurements obtained in the fluoroscopic view: 1, V N-CSO; 2, H N-CSO; 3, V RS-CSO; 4, H RS-CSO; 5, V RI-CSO; 6, H RI-CSO; 7, VLA; 8, N -angle. The abbreviations are as in the text.
Statistic analysis
All data are presented as the mean ± 1 SD. The measured parameters were compared between the first and second procedures using the Wilcoxon signed ranks test. The incidence of a difficult puncture was compared between the first and second procedures using a χ2 test. A P -value <0.05 was considered statistically significant.
Results
Compared with the first procedure ( Table 1 ), the vertical distance between the transseptal puncture site and CSO was higher in the second procedure both in the atrial end-systolic phase (34.8 ± 5.3 vs. 38.0 ± 4.7 mm, P = 0.036) and in the end-diastolic phase (39.1 ± 5.4 vs. 43.0 ± 4.8 mm, P = 0.004) ( Figure 2 ). In the end-systolic phase, the horizontal distance between the transseptal puncture site and CSO (8.7 ± 5.8 vs. 9.2 ± 4.7 mm, P = 0.25), vertical distance between the right superior PV–left atrial junction and CSO (58.0 ± 7.1 vs. 58.1 ± 7.9 mm, P = 0.97), horizontal distance between the right superior PV–left atrial junction and CSO (21.6 ± 9.3 vs. 21.8 ± 8.9 mm, P = 0.91), vertical distance between the right inferior PV–left atrial junction and CSO (30.0 ± 8.5 vs. 28.7 ± 7.0 mm, P = 0.29), horizontal distance between the right inferior PV–left atrial junction and CSO (28.9 ± 11.1 vs. 30.7 ± 9.5 mm, P = 0.57), longest vertical distance between the roof and bottom of the left atrium (43.4 ± 8.8 vs. 43.0 ± 6.8 mm, P = 0.96), and angle between the direction of needle at the puncture site and horizontal line (57.2 ± 5.1° vs. 57.2 ± 4.9°, P = 0.64) exhibited no differences between the first and second sessions.
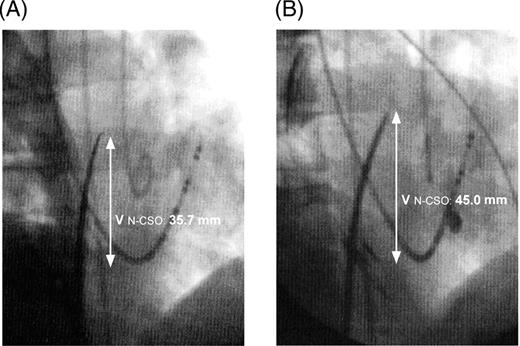
The successful puncture site in the first procedure ( A ) was lower than that in the second procedure ( B ) during the atrial end-diastolic phase.
| . | First procedure . | Second procedure . | P -value . |
|---|---|---|---|
| Atrial end-systolic phase | |||
| V N-CSO, mm | 34.8 ± 5.3 | 38.0 ± 4.7 | 0.036 |
| Atrial end-diastolic phase | |||
| V N-CSO, mm | 39.1 ± 5.4 | 43.0 ± 4.8 | 0.004 |
| H N-CSO, mm | 12.0 ± 5.9 | 12.5 ± 5.2 | 0.33 |
| V RS-CSO, mm | 62.6 ± 5.9 | 64.2 ± 6.7 | 0.34 |
| H RS-CSO, mm | 28.2 ± 8.9 | 27.8 ± 9.3 | 0.67 |
| V RI-CSO, mm | 31.0 ± 8.2 | 32.7 ± 6.3 | 0.19 |
| H RI-CSO, mm | 35.7 ± 9.9 | 36.5 ± 9.4 | 0.12 |
| VLA, mm | 55.3 ± 7.6 | 54.0 ± 5.5 | 0.46 |
| N -angle | 58.2 ± 5.7 | 58.3 ± 5.2 | 0.64 |
| . | First procedure . | Second procedure . | P -value . |
|---|---|---|---|
| Atrial end-systolic phase | |||
| V N-CSO, mm | 34.8 ± 5.3 | 38.0 ± 4.7 | 0.036 |
| Atrial end-diastolic phase | |||
| V N-CSO, mm | 39.1 ± 5.4 | 43.0 ± 4.8 | 0.004 |
| H N-CSO, mm | 12.0 ± 5.9 | 12.5 ± 5.2 | 0.33 |
| V RS-CSO, mm | 62.6 ± 5.9 | 64.2 ± 6.7 | 0.34 |
| H RS-CSO, mm | 28.2 ± 8.9 | 27.8 ± 9.3 | 0.67 |
| V RI-CSO, mm | 31.0 ± 8.2 | 32.7 ± 6.3 | 0.19 |
| H RI-CSO, mm | 35.7 ± 9.9 | 36.5 ± 9.4 | 0.12 |
| VLA, mm | 55.3 ± 7.6 | 54.0 ± 5.5 | 0.46 |
| N -angle | 58.2 ± 5.7 | 58.3 ± 5.2 | 0.64 |
The abbreviations are as in the text.
| . | First procedure . | Second procedure . | P -value . |
|---|---|---|---|
| Atrial end-systolic phase | |||
| V N-CSO, mm | 34.8 ± 5.3 | 38.0 ± 4.7 | 0.036 |
| Atrial end-diastolic phase | |||
| V N-CSO, mm | 39.1 ± 5.4 | 43.0 ± 4.8 | 0.004 |
| H N-CSO, mm | 12.0 ± 5.9 | 12.5 ± 5.2 | 0.33 |
| V RS-CSO, mm | 62.6 ± 5.9 | 64.2 ± 6.7 | 0.34 |
| H RS-CSO, mm | 28.2 ± 8.9 | 27.8 ± 9.3 | 0.67 |
| V RI-CSO, mm | 31.0 ± 8.2 | 32.7 ± 6.3 | 0.19 |
| H RI-CSO, mm | 35.7 ± 9.9 | 36.5 ± 9.4 | 0.12 |
| VLA, mm | 55.3 ± 7.6 | 54.0 ± 5.5 | 0.46 |
| N -angle | 58.2 ± 5.7 | 58.3 ± 5.2 | 0.64 |
| . | First procedure . | Second procedure . | P -value . |
|---|---|---|---|
| Atrial end-systolic phase | |||
| V N-CSO, mm | 34.8 ± 5.3 | 38.0 ± 4.7 | 0.036 |
| Atrial end-diastolic phase | |||
| V N-CSO, mm | 39.1 ± 5.4 | 43.0 ± 4.8 | 0.004 |
| H N-CSO, mm | 12.0 ± 5.9 | 12.5 ± 5.2 | 0.33 |
| V RS-CSO, mm | 62.6 ± 5.9 | 64.2 ± 6.7 | 0.34 |
| H RS-CSO, mm | 28.2 ± 8.9 | 27.8 ± 9.3 | 0.67 |
| V RI-CSO, mm | 31.0 ± 8.2 | 32.7 ± 6.3 | 0.19 |
| H RI-CSO, mm | 35.7 ± 9.9 | 36.5 ± 9.4 | 0.12 |
| VLA, mm | 55.3 ± 7.6 | 54.0 ± 5.5 | 0.46 |
| N -angle | 58.2 ± 5.7 | 58.3 ± 5.2 | 0.64 |
The abbreviations are as in the text.
In the end-diastolic phase, the horizontal distance between the transseptal puncture site and CSO (12.0 ± 5.9 vs. 12.5 ± 5.2 mm, P = 0.33), vertical distance between the right superior PV–left atrial junction and CSO (62.6 ± 5.9 vs. 64.2 ± 6.7 mm, P = 0.34), horizontal distance between the right superior PV–left atrial junction and CSO (28.2 ± 8.9 vs. 27.8 ± 9.3 mm, P = 0.67), vertical distance between the right inferior PV–left atrial junction and CSO (31.0 ± 8.2 vs. 32.7 ± 6.3 mm, P = 0.19), horizontal distance between the right inferior PV–left atrial junction and CSO (35.7 ± 9.9 vs. 36.5 ± 9.4 mm, P = 0.12), longest vertical distance between the roof and bottom of the left atrium (55.3 ± 7.6 vs. 54.0 ± 5.5 mm, P = 0.46), and angle between the direction of the needle at the puncture site and horizontal line (58.3 ± 5.2° vs. 58.2 ± 5.7°, P = 0.64) exhibited no significant differences.
During the first procedure, the large-curved transseptal needles were used in two patients (7%). During the second procedure, large-curved needles were used instead of small-curved needles in six patients. During the first procedure, the large-curved transseptal needles were used in two patients, and the large-curved transseptal needles were still needed during the second procedure. A higher incidence of difficult punctures was noted during the second procedure than in the first procedure (21 vs. 7%, P = 0.037). Although three attempts using the small-curved needles failed in those eight patients, a successful transseptal puncture was achieved after changing to the large-curved needles. The differences between easy and difficult punctures were analysed ( Table 2 ). No differences of age (50.0 ± 10.8 vs.59.1 ± 14.6 years, P = 0.76), male (71.4 vs. 62.5%, P = 0.68), AF duration (5.2 ± 3.8 vs. 5.0 ± 3.4 years, P = 0.85), interval between first and second procedures (8.0 ± 7.3 vs. 7.1 ± 4.3 months, P = 0.75), procedure time of the first procedure (87.7 ± 37.1 vs. 89.0 ± 14.1 min, P = 0.91), and left atrial anteroposterior diameter by transthoracic echocardiography (38.3 ± 5.9 vs. 38.7 ± 6.3 mm, P = 0.89) were noted.
Comparisons between the easy and difficult transseptal procedures in the second ablation procedure
| . | Easy puncture . | Difficult puncture . | P -value . |
|---|---|---|---|
| Number | 21 | 8 | |
| Age (years) | 50.0 ± 10.8 | 59.1 ± 14.6 | 0.76 |
| Male (%) | 71.4 | 62.5 | 0.68 |
| AF duration (years) | 5.2 ± 3.8 | 5.0 ± 3.4 | 0.85 |
| Interval between procedures (months) | 8.0 ± 7.3 | 7.1 ± 4.3 | 0.75 |
| Procedure time of the first procedure (min) | 87.7 ± 37.1 | 89.0 ± 14.1 | 0.91 |
| Left atrial anteroposterior diameter (mm) | 38.3 ± 5.9 | 38.7 ± 6.3 | 0.89 |
| . | Easy puncture . | Difficult puncture . | P -value . |
|---|---|---|---|
| Number | 21 | 8 | |
| Age (years) | 50.0 ± 10.8 | 59.1 ± 14.6 | 0.76 |
| Male (%) | 71.4 | 62.5 | 0.68 |
| AF duration (years) | 5.2 ± 3.8 | 5.0 ± 3.4 | 0.85 |
| Interval between procedures (months) | 8.0 ± 7.3 | 7.1 ± 4.3 | 0.75 |
| Procedure time of the first procedure (min) | 87.7 ± 37.1 | 89.0 ± 14.1 | 0.91 |
| Left atrial anteroposterior diameter (mm) | 38.3 ± 5.9 | 38.7 ± 6.3 | 0.89 |
Comparisons between the easy and difficult transseptal procedures in the second ablation procedure
| . | Easy puncture . | Difficult puncture . | P -value . |
|---|---|---|---|
| Number | 21 | 8 | |
| Age (years) | 50.0 ± 10.8 | 59.1 ± 14.6 | 0.76 |
| Male (%) | 71.4 | 62.5 | 0.68 |
| AF duration (years) | 5.2 ± 3.8 | 5.0 ± 3.4 | 0.85 |
| Interval between procedures (months) | 8.0 ± 7.3 | 7.1 ± 4.3 | 0.75 |
| Procedure time of the first procedure (min) | 87.7 ± 37.1 | 89.0 ± 14.1 | 0.91 |
| Left atrial anteroposterior diameter (mm) | 38.3 ± 5.9 | 38.7 ± 6.3 | 0.89 |
| . | Easy puncture . | Difficult puncture . | P -value . |
|---|---|---|---|
| Number | 21 | 8 | |
| Age (years) | 50.0 ± 10.8 | 59.1 ± 14.6 | 0.76 |
| Male (%) | 71.4 | 62.5 | 0.68 |
| AF duration (years) | 5.2 ± 3.8 | 5.0 ± 3.4 | 0.85 |
| Interval between procedures (months) | 8.0 ± 7.3 | 7.1 ± 4.3 | 0.75 |
| Procedure time of the first procedure (min) | 87.7 ± 37.1 | 89.0 ± 14.1 | 0.91 |
| Left atrial anteroposterior diameter (mm) | 38.3 ± 5.9 | 38.7 ± 6.3 | 0.89 |
Discussion
The study demonstrated that the puncture site moved higher in the second procedure, compared with the first procedure ( Figure 1 A and B ). A higher incidence of a difficult puncture was noted during the second procedure than in the first procedure.
Atrial remodelling
The possible relationship between recurrent AF and atrial dilatation remains controversial. 14–17 The increasing left atrium diameters might be associated with an increased inter-atrial septal diameter, thus changing the position of the foramen ovale, which might result in a higher transseptal puncture site in the second procedure. However, in the present study, the vertical diameter of the left atrium did not change between the procedures. The vertical and horizontal distances between the right superior PV and CSO exhibited no significant differences, and that was also the case for the right inferior PV. The atrial dilatation cannot explain the need for the higher transseptal puncture site during the second procedure.
Local scarring and difficult puncture
Marcus et al . 5 reported that simple catheter manipulation across the transseptal puncture site over an extended period of time may have produced enough irritation to cause inflammation and chronic scarring (as evidenced by the patent foramen ovale closures occurring in patients with longer transseptal times). Local scarring over the puncture site during the first procedure may result in a difficult puncture over the same area and nearby areas during the second procedure. Hanaoka et al . 18 used intracardiac echocardiography to evaluate the transseptal puncture site in 19 patients undergoing radiofrequency catheter ablation. The puncture site tended to shift upwards and around the upper edge of the fossa ovalis in 17 of those patients (89%). In this study, a higher incidence of difficult punctures was noted during the second procedure than in the first procedure, which may be due to local scarring over the previous puncture site. The transseptal needle tended to shift upwards skipping over the scar area during the second procedure, which would explain the higher vertical distance between the transseptal puncture site and CSO. Using the small-curved needle, the tip of the needle was continuously slipping upwards, and therefore we could not find an appropriate transseptal puncture site. After changing to the large-curved needle design, the slipping further upwards of the puncture site decreased, and the transseptal procedure was successful. Although further large-scale trials are needed, the large-curved transseptal needles should be suggested instead of the small-curved needles during the second procedure.
Longer duration of the first procedure may increase the irritation of septum, promote more scar, and increase the difficulty in the second procedure. There is the possibility that the patients with short interval between the first and the second procedure may not provide the same issues as those with longer time allowing full scarring to develop. In the present study, the procedure time and the time intervals between the first and second procedures were not different between easy and difficult punctures. Similar results were reported by Marcus et al . 5 Moreover, no difference of atrial size was noted, which means the difficulty was related to local scar rather than left atrial enlargement.
Limitations
The measurements of the distance between each landmark may not accurately reflect the true anatomical distances. The relationships of each landmark are three-dimensional, and this study calculated the distance on the basis of the two-dimensional fluoroscopic view. The measurements of each parameter might change during the different cardiac and respiratory cycles. The differences in magnification and angulations between patients or between studies might affect the measurements. But such measurement errors would be expected to have affected all measurements and yet only VN-CSO differed significantly between studies. Intra-observer variability in making these measurements could lead to bias.
Moreover, in our laboratory, Lasso catheter and the ablation catheter were placed through the same puncture hole. One might hypothesize that two separate punctures might stimulate more fibrosis than two catheters delivered through one puncture site. Further study was needed to answer these questions. A larger population from different centres will be necessary in the future studies to prove this finding.
Conclusions
The incidence of a difficult puncture was higher in the second procedure compared with the first procedure. The transseptal puncture site moved higher in the second procedure. Chronic scarring over the previous transseptal site is a reasonable hypothesis to explain the observations. The difficult punctures experienced during the second procedure might be overcome by changing the needle curve from a small curve to a large curve design.
Conflict of interest : none declared.



