-
PDF
- Split View
-
Views
-
Cite
Cite
Renato Pietro Ricci, Loredana Morichelli, Massimo Santini, Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: impact on medical management and health-care resource utilization, EP Europace, Volume 10, Issue 2, February 2008, Pages 164–170, https://doi.org/10.1093/europace/eum289
Close - Share Icon Share
Abstract
To evaluate the impact of Home Monitoring™(HM) remote control on patient medical treatment and on health-care resource utilization.
One hundred and seventeen patients received HM pacemakers or defibrillators. A pacing expert nurse consulted daily the website and submitted critical cases to physician. During a mean follow-up of 227 ± 128 days, 25 210 messages were received (23 545 daily messages and 1665 alert events) resulting in 90.7% of HM supervised days. Fifty-nine minutes/week for the nurse and 12 min/week for the physician were spent for HM data analysis during 267 web-connections. The mean connection time per patient was 115 ± 60 s. The nurse submitted to the physician 133 critical cases in 56 patients. The diagnosis were atrial fibrillation (47%), ventricular tachyarrhythmias (9%), inappropriate implantable cardioverter defibrillator intervention (4%), unsustained ventricular tachycardia (7%), device suboptimal programming (23%), and impending heart failure (10%). Sixty-six unplanned follow-up in 43 patients led to drug therapy change (44%), device reprogramming (18%), diagnosis confirmation without further intervention (24%), no confirmation (6%), further diagnostic tests (9%).
HM technology allowed optimization of medical treatment and device programming with low consumption of health-care resource.
Introduction
Pacemakers and implantable cardioverter defibrillators (ICDs) equipped with the wireless Home Monitoring™ function (HM, Biotronik GmbH & Co. KG, Berlin, Germany) are capable of providing on daily basis automatic information about the patient cardiac condition as well as the implant status. 1 Devices have an embedded antenna for wireless transmissions of diagnostic information to a Service Center where messages are decrypted, stored as well as loaded on a protected website accessible to the attending physician through identity codes and a personal password.
Several studies have demonstrated the feasibility of this remote monitoring system as well as its technical reliability. 2–4 Potential advantages of HM for patient management include early detection of device technical troubles, early reaction to changes in patient clinical status, such as atrial and ventricular arrhythmia development or heart failure progression, reduction of unnecessary out-patient visits and optimization of health-care resource allocation. 5–7
In spite of so promising benefits of the system, little data is available about the optimal health-care organization as well as about changes induced in the therapeutical approach.
The aim of our study is to evaluate the impact of HM technology on patient medical treatment and on health care resource utilization in a high-volume pacemaker and ICD European clinic.
Methods
Patient population
From April 2006 to June 2007, 117 patients (38 female) of mean age 70.4 ± 10.1 years, received HM devices [88 dual chamber pacemakers, 18 dual chamber ICDs, 11 ICDs combined with cardiac resynchronization therapy (CRT)]. In the pacemaker group implant indications were sinus node disease in 60%, atrio-ventricular or intraventricular blocks in 24%, vasovagal syncope in 13%, and carotid sinus syndrome in 3%. In the ICD group, implant indications were primary prevention of sudden cardiac death in 34% and secondary prevention in 66%. Clinical characteristics of enrolled patients are summarized in Table 1 .
| . | PM ( n = 88) . | ICDs ( n = 29) . |
|---|---|---|
| Age (years) | 74.5 ± 8.0 | 62.0 ± 14.8 |
| Male (%) | 62 | 86 |
| PM implant indication | ||
| SSS (%) | 53 (60) | |
| AV block (%) | 21 (24) | |
| Neuromediate syncope (%) | 14 (16) | |
| Ejection fraction % | 37.2 ± 14.0 | |
| ICD implant indication (%) | ||
| Primary | 10 (34) | |
| Secondary | 19 (66) | |
| Structural heart disease (%) | ||
| Hypertension | 32 (36) | 2 (7) |
| Hyschemic | 16 (18) | 15 (52) |
| Valvular | 6 (7) | 0 (0) |
| Cardiomyopathy | 2 (2) | 9 (31) |
| None | 32 (36) | 2 (7) |
| Prior atrial fibrillation (%) | 29 (33) | 5 (26) |
| Prior myocardial infarction (%) | 5 (6) | 9 (31) |
| Prior myocardial revascularization (%) | ||
| PCI | 6 (7) | 3 (10) |
| CABG | 1 (1) | 2 (7) |
| Prior RF ablation | 2 (2) | 0 (0) |
| . | PM ( n = 88) . | ICDs ( n = 29) . |
|---|---|---|
| Age (years) | 74.5 ± 8.0 | 62.0 ± 14.8 |
| Male (%) | 62 | 86 |
| PM implant indication | ||
| SSS (%) | 53 (60) | |
| AV block (%) | 21 (24) | |
| Neuromediate syncope (%) | 14 (16) | |
| Ejection fraction % | 37.2 ± 14.0 | |
| ICD implant indication (%) | ||
| Primary | 10 (34) | |
| Secondary | 19 (66) | |
| Structural heart disease (%) | ||
| Hypertension | 32 (36) | 2 (7) |
| Hyschemic | 16 (18) | 15 (52) |
| Valvular | 6 (7) | 0 (0) |
| Cardiomyopathy | 2 (2) | 9 (31) |
| None | 32 (36) | 2 (7) |
| Prior atrial fibrillation (%) | 29 (33) | 5 (26) |
| Prior myocardial infarction (%) | 5 (6) | 9 (31) |
| Prior myocardial revascularization (%) | ||
| PCI | 6 (7) | 3 (10) |
| CABG | 1 (1) | 2 (7) |
| Prior RF ablation | 2 (2) | 0 (0) |
AV, atrio-ventricular; PM, pacemaker; ICD, implantable cardioverter defibrillator; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; RF, radiofrequency.
| . | PM ( n = 88) . | ICDs ( n = 29) . |
|---|---|---|
| Age (years) | 74.5 ± 8.0 | 62.0 ± 14.8 |
| Male (%) | 62 | 86 |
| PM implant indication | ||
| SSS (%) | 53 (60) | |
| AV block (%) | 21 (24) | |
| Neuromediate syncope (%) | 14 (16) | |
| Ejection fraction % | 37.2 ± 14.0 | |
| ICD implant indication (%) | ||
| Primary | 10 (34) | |
| Secondary | 19 (66) | |
| Structural heart disease (%) | ||
| Hypertension | 32 (36) | 2 (7) |
| Hyschemic | 16 (18) | 15 (52) |
| Valvular | 6 (7) | 0 (0) |
| Cardiomyopathy | 2 (2) | 9 (31) |
| None | 32 (36) | 2 (7) |
| Prior atrial fibrillation (%) | 29 (33) | 5 (26) |
| Prior myocardial infarction (%) | 5 (6) | 9 (31) |
| Prior myocardial revascularization (%) | ||
| PCI | 6 (7) | 3 (10) |
| CABG | 1 (1) | 2 (7) |
| Prior RF ablation | 2 (2) | 0 (0) |
| . | PM ( n = 88) . | ICDs ( n = 29) . |
|---|---|---|
| Age (years) | 74.5 ± 8.0 | 62.0 ± 14.8 |
| Male (%) | 62 | 86 |
| PM implant indication | ||
| SSS (%) | 53 (60) | |
| AV block (%) | 21 (24) | |
| Neuromediate syncope (%) | 14 (16) | |
| Ejection fraction % | 37.2 ± 14.0 | |
| ICD implant indication (%) | ||
| Primary | 10 (34) | |
| Secondary | 19 (66) | |
| Structural heart disease (%) | ||
| Hypertension | 32 (36) | 2 (7) |
| Hyschemic | 16 (18) | 15 (52) |
| Valvular | 6 (7) | 0 (0) |
| Cardiomyopathy | 2 (2) | 9 (31) |
| None | 32 (36) | 2 (7) |
| Prior atrial fibrillation (%) | 29 (33) | 5 (26) |
| Prior myocardial infarction (%) | 5 (6) | 9 (31) |
| Prior myocardial revascularization (%) | ||
| PCI | 6 (7) | 3 (10) |
| CABG | 1 (1) | 2 (7) |
| Prior RF ablation | 2 (2) | 0 (0) |
AV, atrio-ventricular; PM, pacemaker; ICD, implantable cardioverter defibrillator; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; RF, radiofrequency.
Home monitoring main features
Implanted devices are equipped with an antenna for long distance telemetry enabling the wireless, automatic, patient-independent transmission of diagnostic information stored in their memory. The transmitter device, namely Cardiomessenger, comprises both features of daily retrieving data from the implanted device through a wireless receiver for long-distance telemetry and forwarding such information to a unique Service Centre placed in Berlin, Germany, by connecting to the GSM (Global System for Mobile Communication) network. The Service Center anonymously decodes, analyses and organizes the data and posts them on a secure website. For each patient trigger events can be pre-selected on clinical basis in order to make medical staff aware as soon as they occur. In fact, in case of events, the Service Center sends sms messages, e-mail and/or faxes either to the attending clinical personnel. Critical event reports for pacemakers include integrity system alerts and diagnostic data on heart rhythm, atrial, and ventricular arrhythmias. For dual-chamber ICDs report triggers for arrhythmia detection and device therapy may also be selected. For CRT–ICDs specific triggers for CRT pacing percentage and heart failure monitoring are available.
Device programming
Pacemakers and ICDs were programmed considering the patient clinical status and according to clinical practice. In all pacemaker patients, the mode switch function was switched on with an intervention rate of 160 bpm, an automatic function for intrinsic rhythm support was activated whenever atrio-ventricular conduction was preserved (PR interval ≤250 ms) and an intracardiac electrogram (IEGM) recording function was activated for atrial and ventricular arrhythmia detection. All the patients were provided with the Cardiomessenger and received instructions for use. All enrolled patients provided written informed consent on HM system utilization and data management.
Moreover, HM features were programmed to daily transmit data at night and to send event report whenever one of the critical events reported in Table 2 occurred. Settings for event reports could be modified during the follow-up to assure an optimal warning and data control for each patient.
| Pacemaker . | Dual-chamber ICD . | ICD–CRT . |
|---|---|---|
| Technical data | Technical data | Technical data |
| Elective replacement indicator | Elective replacement indicator | Elective replacement indicator |
| A and/or V lead impedance <200 Ω and >3000 Ω | A and/or V lead impedance <250 Ω and >1500 Ω | A, right and left V lead impedance <250 Ω and >1500 Ω |
| Increased ventricular threshold ≥4.8 V | Shock impedance <25 Ω and >110 Ω | Biventricular pacing lead impedance <200 Ω and >750 Ω |
| Disabled ventricular capture control | Abnormal system status | Shock impedance <25 Ω and >110 Ω |
| Reduced P or R wave sensing by 50% of safety margin | Arrhythmias episodes dignostic | Abnormal system status |
| Heart Rhythm | VT1, VF, and SVT detection | Arrhythmias episodes diagnostic |
| MS episode duration ≥10% (2, 5 h) | 30 J ineffective shock | VT1, VF, and SVT detection |
| V run (4–8 consecutive VES) | Heart rhythm | 30 J ineffective shock |
| Unsustained VT (>8 consecutive VES) | MS episode duration ≥10% (2, 5 h) | CHF diagnostics |
| V intrinsic rhythm <90% | % CRT pacing <90% | |
| Mean VES/h >100 | MS episode duration ≥10% (2, 5 h) | |
| Mean V rate at rest >80 bpm | ||
| Mean VES/h >100 |
| Pacemaker . | Dual-chamber ICD . | ICD–CRT . |
|---|---|---|
| Technical data | Technical data | Technical data |
| Elective replacement indicator | Elective replacement indicator | Elective replacement indicator |
| A and/or V lead impedance <200 Ω and >3000 Ω | A and/or V lead impedance <250 Ω and >1500 Ω | A, right and left V lead impedance <250 Ω and >1500 Ω |
| Increased ventricular threshold ≥4.8 V | Shock impedance <25 Ω and >110 Ω | Biventricular pacing lead impedance <200 Ω and >750 Ω |
| Disabled ventricular capture control | Abnormal system status | Shock impedance <25 Ω and >110 Ω |
| Reduced P or R wave sensing by 50% of safety margin | Arrhythmias episodes dignostic | Abnormal system status |
| Heart Rhythm | VT1, VF, and SVT detection | Arrhythmias episodes diagnostic |
| MS episode duration ≥10% (2, 5 h) | 30 J ineffective shock | VT1, VF, and SVT detection |
| V run (4–8 consecutive VES) | Heart rhythm | 30 J ineffective shock |
| Unsustained VT (>8 consecutive VES) | MS episode duration ≥10% (2, 5 h) | CHF diagnostics |
| V intrinsic rhythm <90% | % CRT pacing <90% | |
| Mean VES/h >100 | MS episode duration ≥10% (2, 5 h) | |
| Mean V rate at rest >80 bpm | ||
| Mean VES/h >100 |
A, atrial; CRT, cardiac resynchronization therapy; MS, mode switch; SVT, supraventricular tachycardia; V, ventricular; VES, ventricular extrasistole; VF, ventricular fibrillation; VT, ventricular tachycardia.
| Pacemaker . | Dual-chamber ICD . | ICD–CRT . |
|---|---|---|
| Technical data | Technical data | Technical data |
| Elective replacement indicator | Elective replacement indicator | Elective replacement indicator |
| A and/or V lead impedance <200 Ω and >3000 Ω | A and/or V lead impedance <250 Ω and >1500 Ω | A, right and left V lead impedance <250 Ω and >1500 Ω |
| Increased ventricular threshold ≥4.8 V | Shock impedance <25 Ω and >110 Ω | Biventricular pacing lead impedance <200 Ω and >750 Ω |
| Disabled ventricular capture control | Abnormal system status | Shock impedance <25 Ω and >110 Ω |
| Reduced P or R wave sensing by 50% of safety margin | Arrhythmias episodes dignostic | Abnormal system status |
| Heart Rhythm | VT1, VF, and SVT detection | Arrhythmias episodes diagnostic |
| MS episode duration ≥10% (2, 5 h) | 30 J ineffective shock | VT1, VF, and SVT detection |
| V run (4–8 consecutive VES) | Heart rhythm | 30 J ineffective shock |
| Unsustained VT (>8 consecutive VES) | MS episode duration ≥10% (2, 5 h) | CHF diagnostics |
| V intrinsic rhythm <90% | % CRT pacing <90% | |
| Mean VES/h >100 | MS episode duration ≥10% (2, 5 h) | |
| Mean V rate at rest >80 bpm | ||
| Mean VES/h >100 |
| Pacemaker . | Dual-chamber ICD . | ICD–CRT . |
|---|---|---|
| Technical data | Technical data | Technical data |
| Elective replacement indicator | Elective replacement indicator | Elective replacement indicator |
| A and/or V lead impedance <200 Ω and >3000 Ω | A and/or V lead impedance <250 Ω and >1500 Ω | A, right and left V lead impedance <250 Ω and >1500 Ω |
| Increased ventricular threshold ≥4.8 V | Shock impedance <25 Ω and >110 Ω | Biventricular pacing lead impedance <200 Ω and >750 Ω |
| Disabled ventricular capture control | Abnormal system status | Shock impedance <25 Ω and >110 Ω |
| Reduced P or R wave sensing by 50% of safety margin | Arrhythmias episodes dignostic | Abnormal system status |
| Heart Rhythm | VT1, VF, and SVT detection | Arrhythmias episodes diagnostic |
| MS episode duration ≥10% (2, 5 h) | 30 J ineffective shock | VT1, VF, and SVT detection |
| V run (4–8 consecutive VES) | Heart rhythm | 30 J ineffective shock |
| Unsustained VT (>8 consecutive VES) | MS episode duration ≥10% (2, 5 h) | CHF diagnostics |
| V intrinsic rhythm <90% | % CRT pacing <90% | |
| Mean VES/h >100 | MS episode duration ≥10% (2, 5 h) | |
| Mean V rate at rest >80 bpm | ||
| Mean VES/h >100 |
A, atrial; CRT, cardiac resynchronization therapy; MS, mode switch; SVT, supraventricular tachycardia; V, ventricular; VES, ventricular extrasistole; VF, ventricular fibrillation; VT, ventricular tachycardia.
Internet data analysis and management
In order to focus on health care provider organization for HM management we assumed specific guidelines. Our model can be described as follows: an expert nurse dedicated to HM was asked to connect to the HM website for checking data from all patients at least every 15 days and whenever an event report was received. Also the nurse was provided with a dedicated email box and a commercial mobile phone for receiving alerts of event reports from HM service centre. Our guidelines established a specific nurse decision algorithm upon each report analysis: in case of missing messages for more than five consecutive days, the patient had to be contacted by phone for a first check of integrity of the transmission system. In-hospital unscheduled follow-up was required by the nurse if the transmissions were not re-established. In case of observations of critical occurrences or unclear data interpretation, HM reports were submitted by the nurse to the physician who was asked to further evaluate HM files and try a possible diagnosis. After a clinical judgement, when appropriate, the patient was called for an unplanned follow-up either by phone or by an in-hospital visit. Clinical decisions after extra follow-ups could lead either to no further actions, changes in device programming, change in drug therapy, further clinical tests, or even patient hospitalization. Events not confirmed by the follow-up visits were considered as false positive episodes.
A custom software was developed in order to keep track of details about connections to the HM web-site: overall duration of each connection as well as the number and the code of patients analysed were automatically collected. Data was separately collected for nurse and physician connections. Clinical decision after data analysis was automatically stored.
Standard in-hospital follow-up
Standard in hospital follow-up visit intervals were extended due to HM remote control: for ICD patients scheduled follow-up occurred every 6 months (instead of 3 months) and once in a year for pacemaker patients (instead of two visits per year).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. Exact 95% confidence intervals (CI) was calculated for rates and percentage. Comparison between connection times were performed by standard unpaired t -test with P = 0.05 as the significance level.
Results
Follow-up and heart-care resource consumption
The patients were followed on average for 227 ± 128 days (range 3–426 days). Three patients died: the first with a dual chamber ICD after 3 months of follow-up because of stroke, the second one with a dual chamber ICD after 7 months because of kidney failure, and the third with a CRT–ICD after 3 months for refractory heart failure. One patient with a CRT–ICD had his device fully explanted after 1 month because of system infection. Scheduled follow-ups were 114; unscheduled follow-ups due to HM reporting were 53. Of the latter follow-ups, 48 occurred for clinical reasons and 5 for transmission interruptions. A total of 167 in-hospital visits took place. Of note, in an equivalent period, 200 in hospital visits would be expected for a standard follow-up scheduling.
Home monitoring data transmission: technical data
Twenty-five thousand two-hundred and ten reports were received (23 545 daily reports and 1665 event reports). The percentage of monitored days was 0.907 (95% CI, 0.904–0.911) while the percentage of the monitored days per patient was on average 0.914 ± 0.119 (95% CI, 0.680–1.147). More than 74% of patients were monitored for more than 90% of days and more than 91% of patients for more than 70% of days as represented with further detail in the Figure 1 . Three patients with pacemaker had not enough GSM coverage at home so that message transmission was not possible and they were excluded from the study. In 32 patients (26 with pacemaker and 6 with ICD) 55 temporary interruptions of message transmission (≥5 consecutive days) were observed due to the following causes: patients not bringing with them their Cardiomessenger on holidays (10 interruptions, 18%); hospitalizations (8 interruptions, 15%); further technical problems, like inadvertent switching off of the Cardiomessenger or intermittent GSM coverage (37 interruptions, 67%). In three patients, the Cardiomessenger had to be replaced due to dysfunction. In all these cases, regular message transmission could be restored. Internal electrograms stored by the ICD during tachyarrhythmias were correctly transmitted by the HM system in 42/62 (68%) episodes in eight patients.
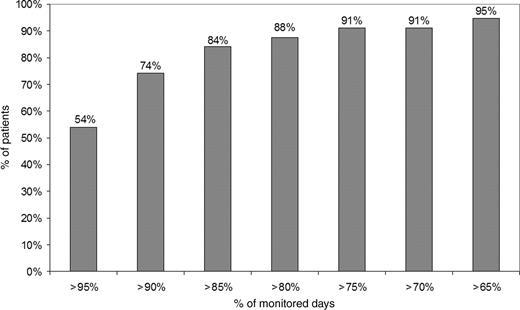
Home monitoring internet connection time
During the follow-up 267 web-connections were performed, 197 by the nurse and 70 by the physician. Overall connection time, as derived by the dedicated counting software, was 69 h and 15 min (57 h and 24 min for the nurse and 11 h and 51 min for the physician). On average, the connection time was 71 min per week, 59 min/week for the nurse and 12 min/week for the physician. The mean connection time per patient was 115 ± 60 s. The connection time for the nurse was significantly lower than the connection time for the physician (96 ± 39 vs. 168 ± 75 s; P < 0.0001). The mean connection time per patient followed a learning curve, showing a significant decrease of 28% over time (from 139 s for the first 50 web-site connections to 99 s for the last 50 web-site connections, P < 0.005).
Home monitoring internet data analysis and clinical interventions
During the 267 web-connections, 2512 entries and analysis of HM patient file were performed, 2249 by the nurse and 263 by the physician. One thousand seven-hundred and twenty-eight analyses were performed in patients with pacemaker, 486 in patients with ICD, and 298 in patients with CRT–ICD. During the follow-up the mean number of analysis per patient was 22 ± 19 (range 1–100). Of the 2249 analysis performed by the nurse, 2061 (92%) were associated with no critical observation and led to no action, 133 (6%) were submitted to the physician for further clinical evaluation, and 55 (2%) needed an additional intervention for restoring transmission interruption (50 phone calls and 5 additional follow-ups).
The physician performed 133 analysis of the HM data in 56 patients (43 pacemaker and 13 ICD). The preliminary diagnosis based on HM was atrial fibrillation in 64 analysis (58 pacemaker and 6 ICD) (47%), sustained ventricular tachycardia or fibrillation in 12 (all ICD) (9%), inappropriate ICD intervention in 5 (4%), unsustained ventricular tachycardia in 9 (8 pacemaker and 1 ICD) (7%), device suboptimal programming in 31 (30 pacemaker and 1 ICD) (23%), and impending heart failure in 13 (1 pacemaker and 12 ICD) (10%). In 50% of the physician's analysis, the HM diagnosis did not lead to further clinical interventions. Forty-three patients (37%) had 66 unplanned contacts (18 consisted in phone calls and 48 in in-hospital visits) leading to the following clinical actions: change in drug therapy in 29 cases (20 pacemaker and 9 ICD) (44%), reprogramming of the device in 12 (8 pacemaker and 4 ICD) (18%), assessment of the appropriateness of the diagnosis without further intervention in 15 (7 pacemaker and 8 ICD) (23%), or of the inappropriateness (false positive) in 4 (3 pacemaker and 1 ICD) (6%), further diagnostic tests needed in 6 (all pacemaker) (9%), hospitalization for impending heart failure in 1 (ICD) (2%).
Arrhythmias and clinical events
Atrial fibrillation
In 33 patients (30 pacemaker and 3 ICD) (28% of the overall population) atrial fibrillation episodes were detected during the follow-up. In 18 patients (55% of those with atrial fibrillation) the arrhythmia was unknown before implant. Sixty-four atrial fibrillation HM reported events were submitted by the nurse to the responsible physician, according to the following criteria: new onset atrial fibrillation, persistent atrial fibrillation, atrial fibrillation burden >10% for more than five consecutive days. In 32 of these events no further action was taken since the patient was judged to be already on optimal treatment. For the remaining 32 events (30 pacemaker and 2 ICD) an unscheduled contact was performed by phone (9 events) and by in-hospital visit (23 events).
After the additional by-phone or in-hospital follow-ups, antiarrhythmic drug therapy was modified in 14 cases (13 pacemaker and 1 ICD) (44% of the unscheduled contacts), anticoagulation was started in 10 (all pacemaker) (31%), and an electrical cardioversion was performed in 3 cases (2 pacemaker and 1 ICD) (9%). Predefined criteria to anticoagulate the patients were the presence of significant clinical risk factors, persistent atrial fibrillation, paroxysmal atrial fibrillation lasting more than 24 h and a mean atrial fibrillation burden higher than 10%. In 4 cases (12%) the device was reprogrammed to avoid intermittent atrial undersensing during atrial fibrillation; in 8 cases (25%) no further action was taken and the ongoing therapy was confirmed. Finally, in 3 cases (9%) the arrhythmia was not confirmed as due to the detection of far field R-wave oversensing (false positive). As an example, Figure 2 shows HM trend data of a patient who developed asymptomatic persistent atrial fibrillation. Home monitoring-based diagnosis allowed to call back the patient to the hospital in order to perform electrical cardioversion. After successful restoration of sinus rhythm, no further episodes of atrial fibrillation were detected during follow-up.
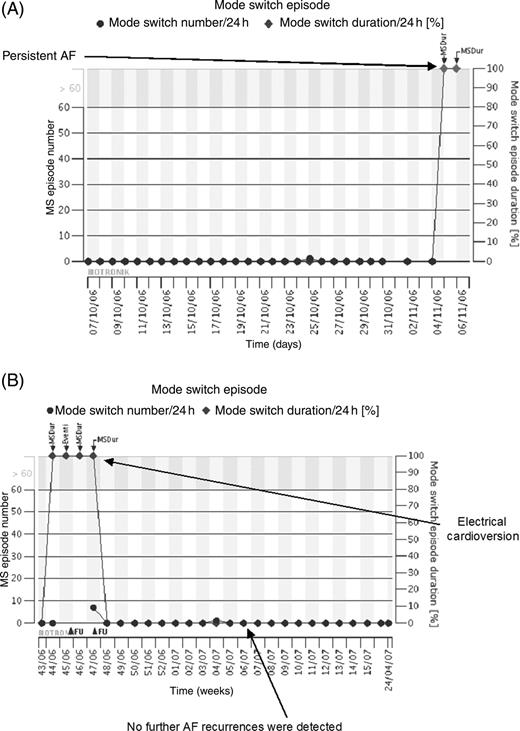
Example of home monitoring trend concerning recurrence of asymptomatic persistent atrial fibrillation. Data are from a 72-year-old woman implanted for Brady–Tachy Syndrome. Circles, number of mode switches; diamonds, mode switch durations as percentage of 24 h. ( A ) After 6 months atrial fibrillation burden suddenly raised from 0 to 100% (persistent atrial fibrillation). The patient was completely asymptomatic. An unscheduled follow-up was considered necessary. ( B ) After an electrical cardioversion was performed and sinus rhythm restored, further atrial fibrillation recurrences were no longer detected.
Ventricular tachycardia/fibrillation
For 8 ICD patients (26% of the ICD patients) IEGM recordings of 42 ventricular arrhythmia episodes were available in the website: 6 episodes in 4 patients were detected as ventricular tachycardia and 36 in 8 patients as ventricular fibrillation. Device detection was appropriate in 32 episodes (4 ventricular tachycardia and 28 ventricular fibrillation). In two cases supraventricular tachycardia was inappropriately detected as ventricular tachycardia; in one case the antiarrhythmic drug therapy was adjusted and in the other case the device was reprogrammed. Inappropriate detection of ventricular fibrillation was due to very fast supraventricular tachycardia entering the ventricular fibrillation detection window in three episodes; the reaction to these events was optimization of drug therapy. In five further cases inappropriate detection of ventricular fibrillation was due to T-wave oversensing. Three episodes were self-limiting while in two episodes inappropriate shocks were delivered. Reprogramming of the device allowed in all cases prevention of further T-wave oversensing episodes. Figure 3 shows an example of self-limiting ventricular fibrillation inappropriate detection due to T-wave oversensing.
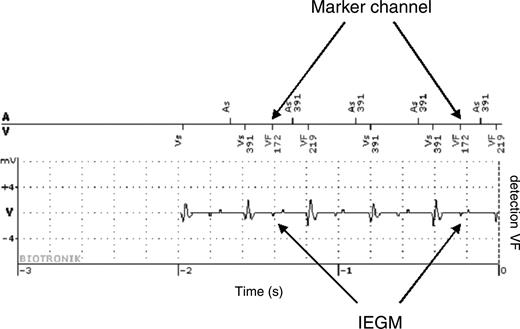
Self-limiting ventricular fibrillation inappropriate detection due to T-wave oversensing. The implantable cardioverter defibrillator was implanted in a 34-year-old male for secondary prevention. Twenty days after discharge a ventricular fibrillation detection occurred not followed by therapy delivery. Arrows indicate T-wave oversensing causing inappropriate detection. No further inappropriate detections occurred after reprogramming of ventricular sensitivity.
Heart failure
In patients with CRT devices a subset of data focused on heart failure status are transmitted on daily basis including ventricular rate at rest, premature ventricular beat frequency, daily activity, and atrial fibrillation burden. Continuous monitoring of these parameters allowed optimization of drug therapy in one patient presenting a sudden increase of mean ventricular rate at rest and premature ventricular beat frequency. In particular, beta blocker therapy dosage was optimized. In another patient, HM indication of very low daily activity and high ventricular rate induced the physician to schedule an hospitalization aimed at the administration of intravenous inotropics.
Discussion
Main study findings
This is the first study that analyses a feasible health care organization in the HM utilization and optimization in routine clinical practice and its impact on patient care. Up to now few studies were conducted mainly to prove the HM data transmission reliability. 1–3 Although these studies proved good performances of HM technology, no information is available on HM clinical utility and on the effects on patient outcome. Our study might give first answers to these topics.
Health-care resource organization
A critical point in introducing device remote control in standard clinical practice is represented by the health-care resource consumption. We proposed a possible organizational model that looked quite attractive. It is based on a close interaction between an expert nurse continuously controlling HM data flow and filtering critical events or unclear interpretations to a responsible physician. This resulted in a great amount of HM data analysis per patient and allowed complete information on the evolution of patient clinical status and device functionality. In spite of this, quite surprisingly, our staff was able to provide a prompt reaction to clinical events with a very short time consumption: the mean connection time per patient was <2 min and the overall connection time was only 71 min per week. Connection time was longer for the physician compared with the nurse probably since the events submitted to the physician required a more complex analysis. In-hospital visits were actually reduced with respect to a standard follow-up scheduling protocol, so that further resource saving may be expected. As a matter of fact, the actual number of in-hospital visits in the HM group (scheduled plus unscheduled) was lower than the number of scheduled visits in our standard clinical practice for a similar patient population. This benefit may be even greater in the long-term follow-up since most of the adverse events usually occur during the first months after implantation and the frequency of HM critical event reporting is expected to decrease over time. This issue, as well as the optimal organizational model to be applied in clinical practice, will have to be evaluated in controlled trials.
Impact of home monitoring on patient care
In our opinion, one of the most important finding of the present study is the demonstration that continuous monitoring of implanted patients through HM technology may deeply impact on their clinical management. In our experience, during a mean follow-up of 7 months, in more than one-third of the patients data from remote control led to major changes in pharmacological treatment or in device programming. As previously reported by Lazarus, 1 the highest percentage of the events revealed by HM are related to atrial fibrillation development, ventricular tachyarrhythmias, ICD interventions, and heart failure.
Concerning atrial fibrillation in pacemaker and ICD patients, it is well known that a large number of them have arrhythmia recurrences during the follow-up, also when there is no history of prior atrial arrhythmias, and that many episodes are asymptomatic. 8 Furthermore, it has been demonstrated that either a high burden of atrial fibrillation, regardless of symptoms, or arrhythmia episodes lasting more than 24 h are independent predictors for stroke and mortality. 9–11 In our population, 28% of patients had atrial fibrillation during the follow-up; the majority of them (91%) had an implanted pacemaker; in 55% of them atrial fibrillation was unknown before implant. At HM-guided unscheduled follow-ups, anticoagulation was started in 30% of them, in 9% an electrical cardioversion was performed, and in 42% antiarrhythmic therapy was modified. An open issue is how to apply guidelines in patient remote control. In other words, it is not known if criteria from standard clinical practice may be directly used in the remote control setting. For instance, which criteria have to be applied to start anticoagulation in patients with atrial fibrillation, specially in patients at low or intermediate risk? Do we have to look at the duration of arrhythmia episodes or at the mean burden of atrial fibrillation? And in the latter case, at which level of burden do we classify a patient being at high risk for stroke? Prospective studies are needed to answer these questions. 12 The ongoing, international, randomized Home-PAT clinical trial is aimed at defining and quantifying the importance of HM for the diagnosis and treatment of atrial fibrillation in patients with dual-chamber pacemakers. 13
Potential benefits of remote control of ICD patients 7 , 14 include early detection of device failure, which may be particularly important for devices in recall, since a daily device status monitoring may allow a ‘wait and see’ strategy, without shortening the in-hospital extra follow-up interval, therefore reducing patient concern and anxiety. Early analysis of ventricular arrhythmia episodes may be of help to physician to refine the therapeutical strategy and to optimize device programming. In our experience, inappropriate detection of supraventricular as ventricular tachycardia induced changes in drug therapy completely resolving the problem without unnecessary patient discomfort. In two episodes, early detection of T-wave oversensing causing false self-limiting ventricular fibrillation episodes allowed early device reprogramming and prevention of inappropriate shock delivery.
In heart failure patients equipped with a cardiac resynchronization therapy device, keeping daily control of actually delivery of resynchronization therapy as well as of heart rate, premature ventricular contractions, daily activity, and atrial and ventricular tachyarrhymias may be of help in optimizing drug therapy, in preventing heart failure progression, and hospitalizations. The potential benefits of the remote control of heart failure patients have been already evaluated in clinical studies enrolling patients without implanted devices. 15 , 16 An on-going trial, the Home CARE trial, 17 is evaluating the clinical impact of HM remote control in patients with heart failure. In our series, strict monitoring of heart failure status allowed optimal beta-blockers titrage, optimization of diuretics and in one case supported the decision to start intravenous inotropics in a severely ill patient non responder to cardiac resynchronization therapy.
Home monitoring data transmission: technical data
The results of the present study confirm the feasibility and technical reliability of the web-based automatic remote control of implanted devices. 1–3 More than 90% of the follow-up days were actually monitored and data were available on daily basis to the referring centre. Sixty-eight percent of IEGM recording upon ventricular tachyarrhythmias occurrences were successfully transmitted showing an exact correspondence with the IEGM strips downloaded at in-hospital follow-up visits. The duration of IEGM episodes HM transmission was always sufficient to obtain a correct diagnosis. The percentage of successful transmission may seem quite low. As a matter of fact most of the unsuccessful IEGM transmission were due to a fast sequence of critical events so as the most recent one withheld and overwrote the transmission procedure of the previous ones. In the next HM generation devices this problem will be overcome by technology improvements.
Study limitations
This is an observational study looking at the impact on standard clinical practice of HM remote control of patients with implanted devices. No randomization between remote control and standard in-hospital follow-up was planned. Criteria to select alert events, to draw changes in drug therapy, and in device programming were defined at the beginning of the study, according to standard clinical practice, but they were someway arbitrary.
Conclusions
This study confirms that HM as an automatic, wireless, internet-based remote control of implanted devices is feasible and reliable. It also may represent a step-up towards a more complete knowledge of the impact of a wide application of this new technology in clinical practice. An organization strategy based on a strict interaction between an expert nurse and a responsible physician was applied requiring very low time consumption and therefore seems to be cost-effective. A prompt advice of device-detected complication as well as arrhythmias and heart failure events allowed optimal patient management and prevention of severe adverse events.
Acknowledgements
The Authors warmly thank Dr Maria Teresa Laudadio, PhD, for her help in data analysis and manuscript review. The authors are grateful to Dr Alessio Gargaro, PhD, for the development of the custom software for Internet connection analysis and for his help in the statistical analysis.
Conflict of interest: none declared.



