-
PDF
- Split View
-
Views
-
Cite
Cite
Fang Zhu, Daniëlle Noordermeer, Elif Aribas, Maxime Bos, Eric Boersma, Maryam Kavousi, Metabolic disorders mediate the relation of miscarriage with cardiovascular diseases, European Journal of Preventive Cardiology, Volume 31, Issue 3, February 2024, Pages 330–336, https://doi.org/10.1093/eurjpc/zwad347
Close - Share Icon Share
Abstract
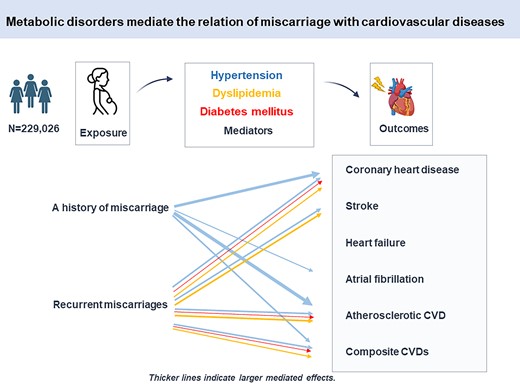
The extent to which the contribution of pregnancy loss to cardiovascular diseases (CVDs) can be explained by metabolic disorders is poorly elucidated but holds insights for reducing long-term cardiovascular risk. The aim of this study is to investigate the mediating effects of hypertension, diabetes mellitus (DM), and lipoprotein metabolism disorders on the association of miscarriage and stillbirth with coronary heart disease (CHD), stroke, heart failure, atrial fibrillation, and composite outcomes.
A total of 163 283 ever-gravid women (age 55.3 ± 7.9 years) from the UK Biobank cohort without established metabolic disorders and CVDs were included and followed from 2007 to 2010 baseline until December 2020. Causal mediation analyses were used to estimate the proportion mediated. Hypertension mediated 11.1% (95% confidence interval, 3.7–18.5%) of the association between a history of miscarriage and incident CHD. Approximately, 9.5% (4.1–14.8%) of the effect of recurrent miscarriages on incident CHD was via hypertension, 8.4% (2.5–14.3%) of the effect was via lipoprotein metabolism disorders, 1.7% (0.5–2.9%) of the effect was via DM, and 10.7% (0.2–21.1%) of the effect of recurrent miscarriages on incident stroke was via hypertension. Hypertension mediated the largest proportion of effect for the atherosclerotic cardiovascular event (15.5% for a history of miscarriage and 9.4% for recurrent miscarriages), followed by lipoprotein metabolism disorders and DM.
Hypertension, DM, and lipoprotein metabolism disorders mediated the association between miscarriage and various cardiovascular outcomes in later life. In particular, hypertension mediated a large proportion of the relationship between miscarriage and atherosclerotic CVD.
Lay Summary
Hypertension, diabetes, and lipoprotein metabolism disorders mediated the association between miscarriage and various cardiovascular outcomes in later life. Hypertension mediated the largest proportion of effect for the atherosclerotic cardiovascular event (15.5% for a history of miscarriage and 9.4% for recurrent miscarriages). Women who have experienced miscarriage should be regularly monitored for possible required interventions on blood pressure, blood lipids, and glucose to reduce their long-term cardiovascular risk.
Our findings contribute to ongoing research efforts to better understand the pathogenesis of pregnancy loss leading to CVD. In particular, we identified metabolic disorders processes as potential mediators.
Implications: Our findings warrant early monitoring and intensive (preventive) treatment of hypertension and lipoprotein metabolism disorders among women who experience miscarriage(s) to lower their burden of later-life clinical cardiovascular events.
Introduction
Pregnancy is viewed as a cardiovascular stress test that can predict women’s cardiovascular health later in life. Miscarriage (spontaneous abortion) is generally defined as the loss of a pregnancy before viability. It is the most common pregnancy complication, with an estimated 23 million miscarriages occurring every year globally.1 Stillbirth refers to pregnancy loss from 24 weeks, with an estimated 2.6 million stillbirths occurring in 2015.2 Both miscarriage and stillbirth could increase the lifetime risk of cardiovascular diseases (CVDs).1,3–5 However, the pathophysiologic mechanisms remain considerably understudied.
Substantial evidence supports the existence of shared predisposing metabolic factors for pregnancy loss and CVD.4,6,7 Metabolic disorders, including hypertension, diabetes mellitus (DM), and dyslipidaemia, are well-established CVD risk factors. They could contribute to vascular stiffness, pro-thrombotic and pro-inflammatory states, and other pregnancy complications such as gestational diabetes and preeclampsia.8,9 Women who experienced pregnancy loss are thought to be at an increased risk of developing metabolic disorders in later life,10,11 possibly through the background of systemic inflammation, endothelial dysfunction, poor preconception metabolic profile, psychological disorders, and unhealthy lifestyles.1,3,7,10,11 In this context, metabolic disorders may be potential mediators of the link between pregnancy loss and later-life CVD.
To date, the extent to which the contribution of pregnancy loss to CVDs can be explained by metabolic disorders is poorly elucidated. Only one recent study observed minor mediating roles of metabolic disorders in the association of pregnancy loss with a combination of coronary heart disease (CHD) and stroke.12 Moreover, it remains unclear (i) how metabolic disorders mediate the association between different types of pregnancy loss (miscarriage and stillbirth) and broader CVD outcomes; (ii) whether metabolic disorders exhibit enhanced mediating effects for recurrent pregnancy loss. Using data from the UK Biobank, we investigated the causal mediating effects of hypertension, DM, and dyslipidaemia on the association of miscarriage and stillbirth with a range of CVD outcomes.
Methods
Study population
The UK Biobank is a prospective population-based cohort that recruited over 500 000 participants aged 37–73 years from 22 assessment centres across England, Scotland, and Wales. Details of the study design have been described previously.13 Through questionnaires, interviews, recurrent visits to assessment centres, and linkage to the health records, a wide range of psychosocial, sociodemographic, physical, and genetic data were collected. Written informed consent was obtained for all participants electronically.
This study sets the baseline for the UK Biobank baseline assessment (2007–10). Among women within the UK Biobank (n = 273 329), we extracted 227 884 ever-gravid women aged between 40 and 70 years with information on pregnancy loss. We excluded 52 948 women with a history of CHD, stroke, heart failure (HF), atrial fibrillation (AF), and metabolic disorders (including DM, hypertension, lipoprotein metabolism disorders, or taking relevant medication) at baseline. Finally, a total of 163 283 participants were included (Table 1).
| Age, years | 55.3 ± 7.9 |
| White ethnicity, % | 148 490 (90.9) |
| Townsend index | −1.6 ± 2.9 |
| Waist, cm | 82.8 ± 11.4 |
| Body mass index, kg/m2 | 26.4 ± 4.7 |
| Low-density lipoprotein cholesterol, mmol/L | 3.7 ± 0.8 |
| Total cholesterol, mmol/L | 6.0 ± 1.1 |
| Glucose, mmol/L | 4.9 ± 0.7 |
| Systolic blood pressure, mmHg | 132.9 ± 18.8 |
| Alcohol intake, % | |
| Daily | 26 910 (16.5) |
| 1–4 times/week | 79 863 (48.9) |
| <1 time/week | 43 487 (26.6) |
| Never | 12 946 (7.9) |
| Smoking status, % | |
| Never | 96 872 (59.5) |
| Former | 50 926 (31.3) |
| Current | 14 968 (9.2) |
| Ever live birth, % | 155 405 (95.2) |
| Age of first live birth, years | 25.74 (4.68) |
| Number of miscarriages, % | |
| 0 | 123 409 (75.6) |
| 1 | 28 773 (17.6) |
| ≥2 | 11 101 (6.8) |
| Number of stillbirths, % | |
| 0 | 159 091 (97.4) |
| 1 | 3665 (2.2) |
| ≥2 | 527 (0.3) |
| Age, years | 55.3 ± 7.9 |
| White ethnicity, % | 148 490 (90.9) |
| Townsend index | −1.6 ± 2.9 |
| Waist, cm | 82.8 ± 11.4 |
| Body mass index, kg/m2 | 26.4 ± 4.7 |
| Low-density lipoprotein cholesterol, mmol/L | 3.7 ± 0.8 |
| Total cholesterol, mmol/L | 6.0 ± 1.1 |
| Glucose, mmol/L | 4.9 ± 0.7 |
| Systolic blood pressure, mmHg | 132.9 ± 18.8 |
| Alcohol intake, % | |
| Daily | 26 910 (16.5) |
| 1–4 times/week | 79 863 (48.9) |
| <1 time/week | 43 487 (26.6) |
| Never | 12 946 (7.9) |
| Smoking status, % | |
| Never | 96 872 (59.5) |
| Former | 50 926 (31.3) |
| Current | 14 968 (9.2) |
| Ever live birth, % | 155 405 (95.2) |
| Age of first live birth, years | 25.74 (4.68) |
| Number of miscarriages, % | |
| 0 | 123 409 (75.6) |
| 1 | 28 773 (17.6) |
| ≥2 | 11 101 (6.8) |
| Number of stillbirths, % | |
| 0 | 159 091 (97.4) |
| 1 | 3665 (2.2) |
| ≥2 | 527 (0.3) |
Data presented as mean ± standard deviation, or number (percentage).
| Age, years | 55.3 ± 7.9 |
| White ethnicity, % | 148 490 (90.9) |
| Townsend index | −1.6 ± 2.9 |
| Waist, cm | 82.8 ± 11.4 |
| Body mass index, kg/m2 | 26.4 ± 4.7 |
| Low-density lipoprotein cholesterol, mmol/L | 3.7 ± 0.8 |
| Total cholesterol, mmol/L | 6.0 ± 1.1 |
| Glucose, mmol/L | 4.9 ± 0.7 |
| Systolic blood pressure, mmHg | 132.9 ± 18.8 |
| Alcohol intake, % | |
| Daily | 26 910 (16.5) |
| 1–4 times/week | 79 863 (48.9) |
| <1 time/week | 43 487 (26.6) |
| Never | 12 946 (7.9) |
| Smoking status, % | |
| Never | 96 872 (59.5) |
| Former | 50 926 (31.3) |
| Current | 14 968 (9.2) |
| Ever live birth, % | 155 405 (95.2) |
| Age of first live birth, years | 25.74 (4.68) |
| Number of miscarriages, % | |
| 0 | 123 409 (75.6) |
| 1 | 28 773 (17.6) |
| ≥2 | 11 101 (6.8) |
| Number of stillbirths, % | |
| 0 | 159 091 (97.4) |
| 1 | 3665 (2.2) |
| ≥2 | 527 (0.3) |
| Age, years | 55.3 ± 7.9 |
| White ethnicity, % | 148 490 (90.9) |
| Townsend index | −1.6 ± 2.9 |
| Waist, cm | 82.8 ± 11.4 |
| Body mass index, kg/m2 | 26.4 ± 4.7 |
| Low-density lipoprotein cholesterol, mmol/L | 3.7 ± 0.8 |
| Total cholesterol, mmol/L | 6.0 ± 1.1 |
| Glucose, mmol/L | 4.9 ± 0.7 |
| Systolic blood pressure, mmHg | 132.9 ± 18.8 |
| Alcohol intake, % | |
| Daily | 26 910 (16.5) |
| 1–4 times/week | 79 863 (48.9) |
| <1 time/week | 43 487 (26.6) |
| Never | 12 946 (7.9) |
| Smoking status, % | |
| Never | 96 872 (59.5) |
| Former | 50 926 (31.3) |
| Current | 14 968 (9.2) |
| Ever live birth, % | 155 405 (95.2) |
| Age of first live birth, years | 25.74 (4.68) |
| Number of miscarriages, % | |
| 0 | 123 409 (75.6) |
| 1 | 28 773 (17.6) |
| ≥2 | 11 101 (6.8) |
| Number of stillbirths, % | |
| 0 | 159 091 (97.4) |
| 1 | 3665 (2.2) |
| ≥2 | 527 (0.3) |
Data presented as mean ± standard deviation, or number (percentage).
Definition of pregnancy loss
Data collection information can be found on the UK Biobank website (https://www.ukbiobank.ac.uk/). At the study baseline visit (2007–10), women were asked for information on spontaneous miscarriages and stillbirths by questionnaires, including ever having had spontaneous miscarriage and stillbirth as well as the number of miscarriages and stillbirths. Termination of pregnancy was not included as miscarriage or stillbirth.
Definition of metabolic disorders and cardiovascular outcomes
Participants were continuously monitored for disease occurrences through linkages with health-related medical records, including primary care data, hospital inpatient data, death register records, and self-reported medical conditions. Metabolic disorders and cardiovascular outcomes were defined using International Classification of Diseases 10th Revision (ICD-10) codes, including hypertension (I10–I13, I15), DM (E10–E14), lipoprotein metabolism disorders (E78), CHD (ICD-10 I21–I25), stroke (I60–I64), HF (I50), and AF (I48). For composite outcomes, atherosclerotic CVD (ASCVD) was the occurrence of either CHD or stroke. Global CVD was the occurrence of either CHD, stroke, HF, or AF. To comply with the temporal order of variables in causal models, only metabolic events that occurred prior to CVD events were recorded to support the hypothesis that metabolic disorders precede CVDs.
We collected medication information at baseline, including blood pressure–lowering medication, insulin, and lipid-lowering medication. Participants taking medications were considered to have relevant metabolic disorder symptoms and were defined as prevalent cases. Participants were followed from the baseline visit until CVD development, death, loss of follow-up, or till the date 31 December 2020.
Measurement of other covariates
Socio-economic status was determined using the Townsend Deprivation Index. Height, weight, and waist were measured. Body mass index was calculated as weight (kg)/[height (m)]2. Blood pressure was taken at baseline using the Omron HEM-7015IT digital blood pressure monitor as the mean of two measurements. Serum lipid concentrations were measured using the Beckman Coulter AU580. Self-reported information on smoking status and alcohol intake was collected. Smoking status was categorized as never, former, and current smokers. Alcohol intake was categorized into the following four groups: daily, 1–4 times per week, <1 time per week, and never. The details of these measurements can be found in the study protocol of the UK Biobank (https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf).
Statistical analysis
We defined two levels of pregnancy loss, namely (i) a history of miscarriage or stillbirth and (ii) recurrent miscarriages or stillbirths. For example, women with ≥1 miscarriage were classified as ‘a history of miscarriage’; women with ≥2 miscarriages were classified as ‘recurrent miscarriages’.
We made a hypothesis about the causal-directed acyclic graph (DAG) (Figure 1) and tested paths. First, the association between pregnancy loss and metabolic disorders was assessed using logistic regression models. The occurrences of hypertension, DM, and lipoprotein metabolism disorders during follow-up were set as outcome variables (dichotomous), and pregnancy loss was set as exposure variables. Second, the association between metabolic disorders and cardiovascular outcomes was assessed using logistic regression models. The occurrences of cardiovascular outcomes during follow-up were set as outcome variables (dichotomous), and occurrences of metabolic disorders were set as exposure variables. Third, we assessed the associations of spontaneous miscarriage and stillbirth with incident cardiovascular outcomes using Cox proportional hazard models. The proportional hazards assumption was tested using the Schoenfeld residuals and the assumption was not violated. All models in the three sections above were adjusted for potential confounders, including baseline age (non-linear; three knots), Townsend index, waist, smoking status, alcohol intake, and metabolic factors at baseline (including systolic blood pressure, low-density lipoprotein and total cholesterol, and glucose).
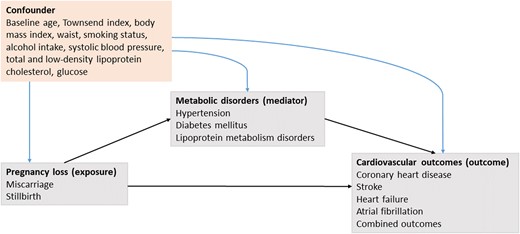
Causal-directed acyclic graph depicting the relations of an assumed causal model.
Next, causal mediation analyses were performed to estimate the proportion of the natural mediating effects of metabolic disorders on the associations under the condition that the three pathways were all valid. We used the method proposed by Li et al.14 which is an extension of the regression-based causal mediation analysis method first proposed by Valeri and VanderWeele.15,16 Models were accounted for the common cause structure (confounding) between mediators and outcomes, including baseline age, Townsend index, body mass index, waist, smoking status, alcohol intake, systolic blood pressure, low-density lipoprotein and total cholesterol, and glucose. We did not include physical activity and age of the first live birth in the main models, which would be potential confounders, mainly due to the high missing rate (>20%). Therefore, as sensitivity analyses, we repeated mediation analyses by additionally adjusting for (i) physical activity and ever had live birth; (ii) physical activity and the age of first live birth. We further added mediation analyses for age-stratified subgroups (age <65 and ≥65 years).
Missing values in covariates ranged between 0.1 and 14.7%; with 77 (0.1%) missing values for alcohol intake, 195 (0.1%) for Townsend index, 440 (0.3%) for waist, 517 (0.3%) for smoking, 603 (0.4%) for body mass index, 9099 (5.6%) for systolic blood pressure, 9632 (5.9%) for total cholesterol, 9884 (6.1%) for low-density lipoprotein cholesterol, and 23 994 (14.7%) for glucose. Missing values in covariates were imputed using the multiple imputation method, and 10 imputed data sets were generated. Analyses were done using R software (regmedint package for mediation analyses, mice package for imputation) with version 4.0.3 (https://www.r-project.org). A two-sided P-value was considered significant at P < 0.05.
Results
Baseline characteristics
A total of 163 283 ever-gravid women (mean ± standard deviation baseline age 55.3 ± 7.9 years) without established metabolic disorders and CVD at baseline were included. Among them, 39 874 (24.4%) women had a history of spontaneous miscarriage (number of miscarriages ≥1), and 11 101 (6.8%) women had experienced recurrent miscarriages; 4192 (2.5%) women had a history of stillbirth (number of stillbirths ≥1), and 527 (0.3%) women had experienced recurrent stillbirths.
Follow-up
During a median of a 12-year follow-up, the number of incident events for CHD, stroke, HF, and AF was 4491, 1762, 1579, and 4334, respectively. The incident rate of global CVD was 5.34 per 1000 person-years. Supplementary material online, Table S1 presents the detailed incident rates for various outcomes.
Mediation pathways
To test the hypothesized pathways (Figure 1), we assessed the association of pregnancy loss with metabolic disorders (Figure 2), the association of metabolic disorders with CVDs (Table 2 and Supplementary material online, Table S1), and the association of pregnancy loss with CVDs (Figure 3). We found only a borderline significant association between a history of stillbirth and lipoprotein metabolism disorders and no significant association between a history of stillbirth and CVD outcomes. A history of miscarriage was found to be related to metabolic disorders and various CVD outcomes. Compared with those without a history of miscarriage, a history of miscarriage was significantly associated with the risk of CHD [hazard ratio (HR), 1.12; 95% confidence interval (CI), 1.05–1.20] and AF (HR, 1.08; 95% CI, 1.01–1.15). Compared with those who did not experience recurrent miscarriages, having experienced recurrent miscarriages was associated with a larger risk of CHD (HR, 1.27; 95% CI, 1.14–1.42) and stroke (HR, 1.20; 95% CI, 1.01–1.43) and a 1.26-fold larger risk of ASCVD (95% CI, 1.15–1.38).
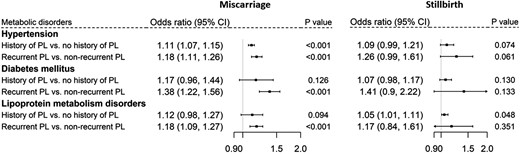
Association of miscarriage (left) and stillbirth (right) with metabolic disorders. PL, pregnancy loss.
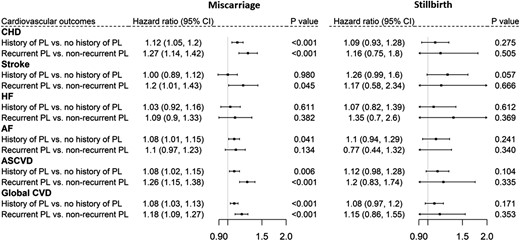
Association of miscarriage (left) and stillbirth (right) with cardiovascular outcomes. PL, pregnancy loss.
Mediating proportion of metabolic disorders on the association between miscarriages and cardiovascular outcomes
| . | A history of miscarriage . | Recurrent miscarriages . | ||||
|---|---|---|---|---|---|---|
| . | Effect (%) . | 95% CI . | P-value . | Effect (%) . | 95% CI . | P-value . |
| Coronary heart disease | ||||||
| Hypertension | 11.1 | 3.7–18.5 | 0.003 | 9.5 | 4.1–14.8 | 0.001 |
| Diabetes mellitus | — | — | — | 1.7 | 0.5–2.9 | 0.004 |
| Lipoprotein metabolism disorders | — | — | — | 8.4 | 2.5–14.3 | 0.005 |
| Stroke | ||||||
| Hypertension | — | — | — | 10.7 | 0.2–21.1 | 0.043 |
| Diabetes mellitus | — | — | — | 1.1 | −0.3 to 2.3 | 0.114 |
| Lipoprotein metabolism disorders | — | — | — | 4.9 | −0.2 to 9.9 | 0.060 |
| Atrial fibrillation | ||||||
| Hypertension | 7.7 | −0.3 to 15.6 | 0.059 | — | — | — |
| Diabetes mellitus | — | — | — | — | — | — |
| Lipoprotein metabolism disorders | — | — | — | — | — | — |
| Atherosclerotic cardiovascular disease | ||||||
| Hypertension | 15.5 | 3.9–27.1 | 0.009 | 9.4 | 4.3–14.5 | <0.001 |
| Diabetes mellitus | — | — | — | 1.5 | 0.5–2.5 | 0.003 |
| Lipoprotein metabolism disorders | — | — | — | 7.9 | 2.7–13.1 | 0.003 |
| Global cardiovascular disease | ||||||
| Hypertension | 10.9 | 3.5–18.2 | 0.004 | 9.3 | 4–14.7 | 0.001 |
| Diabetes mellitus | — | — | — | 1.3 | 0.4–2.1 | 0.004 |
| Lipoprotein metabolism disorders | — | — | — | 6.7 | 2.1–11.4 | 0.004 |
| . | A history of miscarriage . | Recurrent miscarriages . | ||||
|---|---|---|---|---|---|---|
| . | Effect (%) . | 95% CI . | P-value . | Effect (%) . | 95% CI . | P-value . |
| Coronary heart disease | ||||||
| Hypertension | 11.1 | 3.7–18.5 | 0.003 | 9.5 | 4.1–14.8 | 0.001 |
| Diabetes mellitus | — | — | — | 1.7 | 0.5–2.9 | 0.004 |
| Lipoprotein metabolism disorders | — | — | — | 8.4 | 2.5–14.3 | 0.005 |
| Stroke | ||||||
| Hypertension | — | — | — | 10.7 | 0.2–21.1 | 0.043 |
| Diabetes mellitus | — | — | — | 1.1 | −0.3 to 2.3 | 0.114 |
| Lipoprotein metabolism disorders | — | — | — | 4.9 | −0.2 to 9.9 | 0.060 |
| Atrial fibrillation | ||||||
| Hypertension | 7.7 | −0.3 to 15.6 | 0.059 | — | — | — |
| Diabetes mellitus | — | — | — | — | — | — |
| Lipoprotein metabolism disorders | — | — | — | — | — | — |
| Atherosclerotic cardiovascular disease | ||||||
| Hypertension | 15.5 | 3.9–27.1 | 0.009 | 9.4 | 4.3–14.5 | <0.001 |
| Diabetes mellitus | — | — | — | 1.5 | 0.5–2.5 | 0.003 |
| Lipoprotein metabolism disorders | — | — | — | 7.9 | 2.7–13.1 | 0.003 |
| Global cardiovascular disease | ||||||
| Hypertension | 10.9 | 3.5–18.2 | 0.004 | 9.3 | 4–14.7 | 0.001 |
| Diabetes mellitus | — | — | — | 1.3 | 0.4–2.1 | 0.004 |
| Lipoprotein metabolism disorders | — | — | — | 6.7 | 2.1–11.4 | 0.004 |
Models were adjusted for baseline age, Townsend index, body mass index, waist, smoking status, alcohol intake, systolic blood pressure, low-density lipoprotein and total cholesterol, and glucose.
CI, confidence interval.
Mediating proportion of metabolic disorders on the association between miscarriages and cardiovascular outcomes
| . | A history of miscarriage . | Recurrent miscarriages . | ||||
|---|---|---|---|---|---|---|
| . | Effect (%) . | 95% CI . | P-value . | Effect (%) . | 95% CI . | P-value . |
| Coronary heart disease | ||||||
| Hypertension | 11.1 | 3.7–18.5 | 0.003 | 9.5 | 4.1–14.8 | 0.001 |
| Diabetes mellitus | — | — | — | 1.7 | 0.5–2.9 | 0.004 |
| Lipoprotein metabolism disorders | — | — | — | 8.4 | 2.5–14.3 | 0.005 |
| Stroke | ||||||
| Hypertension | — | — | — | 10.7 | 0.2–21.1 | 0.043 |
| Diabetes mellitus | — | — | — | 1.1 | −0.3 to 2.3 | 0.114 |
| Lipoprotein metabolism disorders | — | — | — | 4.9 | −0.2 to 9.9 | 0.060 |
| Atrial fibrillation | ||||||
| Hypertension | 7.7 | −0.3 to 15.6 | 0.059 | — | — | — |
| Diabetes mellitus | — | — | — | — | — | — |
| Lipoprotein metabolism disorders | — | — | — | — | — | — |
| Atherosclerotic cardiovascular disease | ||||||
| Hypertension | 15.5 | 3.9–27.1 | 0.009 | 9.4 | 4.3–14.5 | <0.001 |
| Diabetes mellitus | — | — | — | 1.5 | 0.5–2.5 | 0.003 |
| Lipoprotein metabolism disorders | — | — | — | 7.9 | 2.7–13.1 | 0.003 |
| Global cardiovascular disease | ||||||
| Hypertension | 10.9 | 3.5–18.2 | 0.004 | 9.3 | 4–14.7 | 0.001 |
| Diabetes mellitus | — | — | — | 1.3 | 0.4–2.1 | 0.004 |
| Lipoprotein metabolism disorders | — | — | — | 6.7 | 2.1–11.4 | 0.004 |
| . | A history of miscarriage . | Recurrent miscarriages . | ||||
|---|---|---|---|---|---|---|
| . | Effect (%) . | 95% CI . | P-value . | Effect (%) . | 95% CI . | P-value . |
| Coronary heart disease | ||||||
| Hypertension | 11.1 | 3.7–18.5 | 0.003 | 9.5 | 4.1–14.8 | 0.001 |
| Diabetes mellitus | — | — | — | 1.7 | 0.5–2.9 | 0.004 |
| Lipoprotein metabolism disorders | — | — | — | 8.4 | 2.5–14.3 | 0.005 |
| Stroke | ||||||
| Hypertension | — | — | — | 10.7 | 0.2–21.1 | 0.043 |
| Diabetes mellitus | — | — | — | 1.1 | −0.3 to 2.3 | 0.114 |
| Lipoprotein metabolism disorders | — | — | — | 4.9 | −0.2 to 9.9 | 0.060 |
| Atrial fibrillation | ||||||
| Hypertension | 7.7 | −0.3 to 15.6 | 0.059 | — | — | — |
| Diabetes mellitus | — | — | — | — | — | — |
| Lipoprotein metabolism disorders | — | — | — | — | — | — |
| Atherosclerotic cardiovascular disease | ||||||
| Hypertension | 15.5 | 3.9–27.1 | 0.009 | 9.4 | 4.3–14.5 | <0.001 |
| Diabetes mellitus | — | — | — | 1.5 | 0.5–2.5 | 0.003 |
| Lipoprotein metabolism disorders | — | — | — | 7.9 | 2.7–13.1 | 0.003 |
| Global cardiovascular disease | ||||||
| Hypertension | 10.9 | 3.5–18.2 | 0.004 | 9.3 | 4–14.7 | 0.001 |
| Diabetes mellitus | — | — | — | 1.3 | 0.4–2.1 | 0.004 |
| Lipoprotein metabolism disorders | — | — | — | 6.7 | 2.1–11.4 | 0.004 |
Models were adjusted for baseline age, Townsend index, body mass index, waist, smoking status, alcohol intake, systolic blood pressure, low-density lipoprotein and total cholesterol, and glucose.
CI, confidence interval.
Mediation analysis
Hypertension was the only valid mediator for the link between a history of miscarriage and CVDs in this study. It mediated 11.1% (95% CI, 3.7–18.5%) of the association between a history of miscarriage and incident CHD, 15.5% (95% CI, 3.9–27.1%) of the association for ASCVD, and 10.9% (95% CI, 3.5–18.2%) of the association for global CVD. Hypertension did not show a statistically significant mediating effect on the association between a history of miscarriage and AF (7.7%, 95% CI, −0.3 to 15.6%).
More mediating pathways were involved in the path from recurrent miscarriages to CVDs. All metabolic disorders showed mediating effects on the association of recurrent miscarriages with CHD, stroke, ASCVD, and global CVD. The largest mediating effect was observed for hypertension, followed by lipoprotein metabolism disorders and DM. Approximately, 9.5% (95% CI, 4.1–14.8%) of the effect of recurrent miscarriages on incident CHD was via hypertension, 8.4% (95% CI, 2.5–14.3%) of the effect was via lipoprotein metabolism disorders, 1.7% (95% CI, 0.5–2.9%) of the effect was via DM, 10.7% (95% CI, 0.2–21.1%) of the effect of recurrent miscarriages on incident stroke was via hypertension, and 4.9% (95% CI, −0.2 to 9.9%) of the effect was via lipoprotein metabolism disorders.
Sensitivity analysis
We repeated all mediation analyses by additionally adjusting for potential confounding, including physical activity, ever had live births, and age at first live birth (see Supplementary material online, Tables S3 and S4). After additionally adjusting for physical activity and age at the first live birth, we observed a larger mediating effect for hypertension; up to 13.2% (95% CI, 1.7–24.8%) for the association between a history of miscarriages and global CVD and 10.3% (95% CI, 2.9–17.7%) for the association between recurrent miscarriages and global CVD. In age-stratified subgroup analysis, the mediating effect of metabolic disorders persisted in the age <65 years but lost statistical significance in the older group (≥65 years; see Supplementary material online, Table S5).
Discussion
In this large prospective study of 163 283 women, we found that hypertension, diabetes, and lipoprotein metabolism disorders mediated the association between miscarriage and various cardiovascular outcomes in later life. Hypertension mediated the largest proportion of effect in the relationship between miscarriage and ASCVD (15.5% for a history of miscarriage and 9.4% for recurrent miscarriages). Metabolic disorders did not significantly mediate the link between stillbirth and CVDs.
Limited research has investigated the mediating effect of metabolic disorders on the association between pregnancy loss and CVD. We did not find a mediating role for DM and lipoprotein metabolism disorders in the link between a history of miscarriage and CVDs. However, we observed a large proportion of hypertension-mediated effects (11.1% for CHD and 15.5% for ASCVD). One recent investigation using the Nurses’ Health Study II cohort reported that hypertension, diabetes, and hypercholesterolaemia all exerted minor mediating effects (<1.8%) on the association between miscarriage and ASCVD.12 There are a few explanations for the divergent findings between the two studies. First, the sample size in the current study was substantially larger (>160K vs. 95K) and included participants receiving universal health care. Second, we added baseline-level metabolic factors to the model, which would be potential confounding, which were not adjusted for in the Nurses’ Health Study II. Third, self-reported metabolic disorders were used in the Nurses’ Health Study II cohort and further compared with medical records, which showed high reliability.12 The definition of metabolic disorders in the current study was based on hospital admission records and self-reports using ICD-10, but its accuracy has not been tested in the UK Biobank. We think the onset of metabolic disorders in the UK Biobank could be underestimated, as baseline blood pressure remained relatively high after excluding established cases of hypertension. However, the potential underestimation of metabolic disorders’ onset may reduce our estimated mediating effects, suggesting that the actual mediating impact may be even more prominent.
In addition to hypertension, lipoprotein metabolism disorders moderately mediated the pathway from recurrent miscarriages to subsequent CHD and composite CVDs. The mediating role of DM was less prominent (<2% of the mediation proportion) but nonetheless present. Mechanically, it is assumed that women who experienced miscarriage(s) may have subclinical metabolic abnormalities before pregnancy.7 As such, the pregnancy process exaggerates the adverse effects of these classic cardio-metabolic factors, increasing the risk of pregnancy complications including miscarriage.3 Women suffering from metabolic abnormalities subsequently develop more severe (clinical) disorders and the impact of metabolic abnormalities on vascular dysfunction persists after delivery.3,7 Besides, women who experienced miscarriage(s) were found to have more unfavourable lifestyle behaviour (e.g. heavy alcohol use, poor diet),17 worse socioeconomic status (e.g. income, education),18 and worse psychological status (e.g. depression, anxiety),1,19 which can increase the risk of metabolic disorders as well as CVD later in life. Furthermore, metabolic disorders may be closely related to the shared pathogenesis of pregnancy loss and cardiac dysfunction, including immune disorders, inflammation, and endothelial dysfunction. For instance, endothelial dysfunction might lead to pregnancy loss through placentation-related defects20 and persist after a complicated pregnancy contributing to the development of CVD.21 Meanwhile, lipid deposition22 associated with abnormal lipid profiles and vascular stiffness23 is highly involved in endothelial dysfunction and cardiac dysfunction processes. Notably, we found that metabolic disorders were the main mediators for the association between miscarriage and ASCVD but had limited mediating effect for HF, suggesting that metabolic disorders could exert more impact on atherosclerosis.
Interestingly, hypertension did not have a larger mediating effect in the association between recurrent miscarriages and CVDs, compared with a history of miscarriage. This indicates that women, whether experiencing repeated miscarriages or a single miscarriage, would have similar benefits in CVD risk reduction by controlling blood pressure. We also observed other risk factors, including lipoprotein metabolism disorders and DM, to be involved in the causal link between recurrent miscarriages and cardiovascular outcomes.
We did not find significant associations between stillbirth and cardiovascular outcomes, while other investigations using UK Biobank data showed that stillbirth was associated with a larger CVD risk among women. This might be attributed to our exclusion of women with metabolic disorders at baseline and the low incidence of stillbirths in this study. The UK Biobank cohort did not collect data on the date of pregnancy loss. For those with both pregnancy loss and metabolic disorders before baseline, we could not define temporal sequence, which could lead to problems in the assumed DAG direction. Therefore, we excluded women with metabolic disorders at baseline.
Causal mediation analysis makes several assumptions, including that the exposure(s) and mediator(s) have a causal effect on the outcome.24 We believe that the causal paths outlined in DAGs are plausible, based on previous literature knowledge and associations tested in the analyses. We also took into account the temporal sequences of exposures, mediators, and outcomes. In addition, our research was based on the assumption of no-unmeasured confounding. Although we could not rule out the potential residual confounding, we included the traditional cardiovascular risk factors in our analyses and performed various sets of sensitivity analyses.
Clinical implications
The pathogenesis of pregnancy loss leading to CVD is incompletely understood. Our study highlights metabolic disorders as potential mediators of the link between miscarriage and CVD. These findings warrant early monitoring and intensive (preventive) treatment of hypertension and lipoprotein metabolism disorders among women who experienced miscarriage(s) to lower their burden of later-life clinical cardiovascular events. Future research is needed to elucidate the pathophysiological mechanisms through which metabolic disorders are involved in the link between miscarriage and CVDs, thereby guiding appropriate and timely (preventive) treatment. Furthermore, it is noteworthy that although metabolic disorders partially explained the relationship between pregnancy loss and later CVDs, the residual ‘mediating proportions’ remained large. Further research should explore this gap, add potential mediators such as obesity and inflammation, and explore the intertwined effects of metabolic disorders with social and behavioural factors (e.g. dietary habits, mental health).
Strengths and limitations
The major strengths of this study include the large sample size, prospective design, long follow-up time, and availability of information on pregnancy loss, especially recurrent pregnancy loss data. There are several limitations. First, the study participants were primarily (90.9%) of European ancestry; therefore, whether the findings can be generalized to other ethnic groups requires further investigation. Second, a healthy volunteer selection bias of the UK Biobank has been previously reported.25 We also excluded those with metabolic disorders at baseline, resulting in a healthier study population than a general population. In the light of this, the mediation proportions observed in this research may underestimate the true mediating proportions. Third, although we adjusted for traditional cardiovascular risk factors, they comprised single measurements at baseline of the study, and we could not rule out the possibility of confounding during follow-up (e.g. lifestyle changes, drug therapy) and other unmeasured relevant factors (e.g. mental health, inflammation levels). Fourth, we did not have data on the time of miscarriage and the age of the pregnant woman at the time of miscarriage. Therefore, further research is needed to investigate potential variations in the effects associated with different types of miscarriages.
Conclusions
Hypertension, diabetes, and lipoprotein metabolism disorders mediated the association between miscarriage and various cardiovascular outcomes in later life. In particular, hypertension mediated a large proportion of effect in the relationship between miscarriage and ASCVD. Women who have experienced miscarriage should be regularly monitored for possible required interventions on blood pressure, blood lipids, and glucose to reduce their long-term cardiovascular risk.
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology.
Acknowledgements
The authors are grateful to UK Biobank study participants and staff for making the data available.
Author contribution
F.Z. and M.K. contributed to the conception or design of the work. F.Z., E.B., and M.K. contributed to data acquisition and data interpretation. F.Z. did the statistical analysis and drafted the manuscript. All authors critically revised the manuscript. All of them gave final approval and agreed to be accountable for all aspects of work, thus ensuring integrity and accuracy.
Funding
This study is supported by the Senior Scientist Grant from the Dutch Heart Foundation (03-004-2021-T050). F.Z. is sponsored by Chinese Government Scholarship (20200772001). None of the funders had any role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and in the preparation, review, or approval of the manuscript.
Data availability
The data used in this work were obtained from the UK Biobank (Data Application 58237), which is an open access resource for public health research. The authors declare that all supporting data (e.g. statistical/analytic code) are available upon reasonable request.
References
Author notes
Conflict of interest: none declared.




Comments