-
PDF
- Split View
-
Views
-
Cite
Cite
Alberto Cordero, Belén Alvarez-Alvarez, David Escribano, José Mª García-Acuña, Belén Cid-Alvarez, Moisés Rodríguez-Mañero, Mª Amparo Quintanilla, Rosa Agra-Bermejo, Pilar Zuazola, José R González-Juanatey, Remnant cholesterol in patients admitted for acute coronary syndromes, European Journal of Preventive Cardiology, Volume 30, Issue 4, March 2023, Pages 340–348, https://doi.org/10.1093/eurjpc/zwac286
Close - Share Icon Share
Abstract
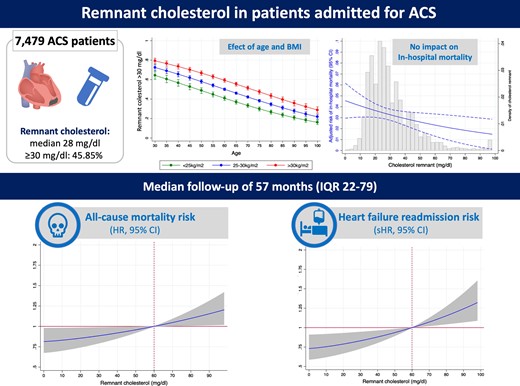
Remnant cholesterol has been identified as one of leading lipid values associated with the incidence of coronary heart disease. There is scarce evidence on its distribution and prognostic value in acute coronary syndrome (ACS) patients.
We included all consecutive patients admitted for ACS in two different centres. Remnant cholesterol was calculated by the equation: total cholesterol minus LDL cholesterol minus HDL cholesterol, and values ≥30 were considered high. Among the 7479 patients, median remnant cholesterol level was 28 mg/dL (21–39), and 3429 (45.85%) patients had levels ≥30 mg/dL. Age (r: −0.29) and body mass index (r: 0.44) were the variables more strongly correlated. At any given age, patients with overweigh or obesity had higher levels. In-hospital mortality was 3.75% (280 patients). Remnant cholesterol was not associated to higher in-hospital mortality risk (odds ratio: 0.89; P = 0.21). After discharge (median follow-up of 57 months), an independent and linear risk of all-cause mortality and heart failure (HF) associated to cholesterol remnant levels was observed. Remnant cholesterol levels >60 mg/dL were associated to higher risk of mortality [hazard ratio (HR): 1.49 95% CI 1.08–2.06; P = 0.016], cardiovascular mortality (HR: 1.49 95% CI 1.08–2.06; P = 0.016), and HF re-admission (sub-HR: 1.55 95% CI 1.14–2.11; P = 0.005).
Elevated remnant cholesterol is highly prevalent in patients admitted for ACS and is inversely correlated with age and positively with body mass index. Remnant cholesterol levels were not associated to higher in-hospital mortality risk, but they were associated with higher long-term risk of mortality and HF.
Lay Summary
Elevated remnant cholesterol is highly prevalent in patients admitted for ACS and is related to body mass index and negatively with age. Remnant cholesterol it is not associated to higher in-hospital mortality risk, but it confers higher long-term risk of mortality and heart failure.
Nearly half of the patients admitted for an acute coronary syndrome have elevated remnant cholesterol.
Remnant cholesterol is associated to higher long-term risk of mortality and heart failure.
Introduction
Despite treatment with most intensive lipid-lowering therapies, the incidence of cardiovascular events and mortality remains high, especially for patients with established cardiovascular disease, and this has been named the lipid residual risk.1,2 A growing body of evidence supports that remnant cholesterol contributes significantly in the incidence of cardiovascular disease beyond the effect of LDL cholesterol (LDLc).3–7
Patients with established cardiovascular disease usually have low levels of HDL cholesterol (HDLc) and intermediate or high triglycerides (TGs). Low levels of high-HDLc,8,9 cholesterol remnants,3,10 or the TGs-to-HDLc (TG/HDLc)11 have been identified as major determinants of acute coronary syndrome (ACS) incidence in low-risk populations. Since there is scarce evidence related to remnant cholesterol distribution and its prognostic impact in patients admitted for ACS, the aim of our study was testing this in large and contemporary cohort of real-world patients.
Methods
We performed a prospective study of all consecutive patients admitted for ACS in two different centres between 2006 and 2016; 5610 (75.11%) from Complejo Hospitalario de la Universidad de Santiago and 1869 (24.99%) from Hospital Universitario de San Juan. A total of 8798 patients were admitted for ACS between November 2003 and December 2016, and 8771 (99.69%) had a lipid profile available for the study. ACS was defined by the presence of typical clinical symptoms of chest pain and electrocardiographic changes indicative of myocardial ischaemia/lesion and/or elevation of serum markers of myocardial damage.12
Remnant cholesterol was calculated by the equation: total cholesterol minus LDLc minus HDLc, and values ≥30 were considered high.3 LDLc was calculated by the Friedewald formula in all patients except when TGs were >200 mg/dL or the value obtained was <40 mg/dL; this happened in 1782 what represent 23.83% of the cohort. Other lipid parameters evaluated were: (i) the estimation of LDL particle size, by the TGs/HDLc (TG/HDL) ratio and values >2 were assumed as low and dense LDL particles13; (ii) total cholesterol/HDLc (TC/HDL) and values >3 were considered high14; (iii) the TGs-to-glucose index (TGGi), obtained as the natural logarithm of (TGs*glucose/2)15; and (iv) non-HDL cholesterol, obtained as total cholesterol minus HDLc.13
ACS was classified as ST-elevation myocardial infarction (STEMI) and non-ST elevation ACS according to the electrocardiographic findings. Mortality risk was assessed by the Global Registry of Acute Coronary Events (GRACE) score,12 and patients were categorized, according to current recommendations, into low (<108), intermediate (109–139), or high risk (>140). Complete revascularization was prospectively determined after the revascularization procedure, on the basis of the intended ‘equivalent anatomic’ revascularization before revascularization on the basis of segment numbering of vessels with a diameter >1.5 mm.16
Risk factors, clinical antecedents, treatments, complementary test, and main diagnosis at discharge were collected by trained medical staff from all patients. The diagnostic and therapeutic ACS protocols in both centres include blood sample determinations in the emergency department and the first fasting state after hospital admission. According to the institution protocols, dyslipidaemia was registered when it was present in medical reports, when patients were under lipid-lowering therapies in absence of indication for previous cardiovascular disease, or if patients were reported to have received the diagnosis of high-cholesterol levels. Glomerular filtration rate was estimated from serum creatinine values with the Chronic Kidney Disease Epidemiology Collaboration equation.17 For the antecedent of previous coronary heart disease, patients needed to have a clinical diagnosis of myocardial infarction, stable or unstable angina, or angina-driven coronary revascularization. Previous heart failure (HF) was codified if patients had at least one hospitalization with such main diagnosis at discharge-medical report as well as those with typical signs and symptoms of HF that had a compatible imagine diagnosis (X-ray or echocardiogram). Comorbidities were assessed by the Charlson index, adapted for patients with cardiovascular disease,18 and patients with Charlson score >4 qualified for high-comorbidity burden. According to current recommendations, optimal medical treatment (OMT) was codified when patients received jointly these four treatments: antiplatelets, statins, beta-blockers, and an angiotensin-converter enzyme inhibitor or angiotensin-receptor blocker.19,20
The post-discharge follow-up of patients has a well-established protocol in each centre and is made by phone calls, review of electronic medical reports, and institutional databases. The vital status was assured by phone calls in absence of medical reports. All health-related processes in health areas are based on electronic resources in both centres. Every time a patient dies, it is registered in patients’ individual electronic medical history by the general practitioner responsible of out-of-hospital care or by a hospital physician, but the status is changed to ‘dead’ only by the department of codification of each health area; therefore, vital status in certified by a double mechanism. HF hospitalization was registered when it was clearly stated in discharge letters. Trained medical staff makes the collection and adjudication of clinical events in both databases. The ethics committee of the coordinator hospital approved the study protocol.
Statistical analyses
Remnant cholesterol had a non-normal distribution, verified by Shapiro–Wilk and Shapiro–Francia tests, and is presented as median (interquartile range). Quantitative variables are presented as mean (SD), and differences were assessed by Student’s t-test and χ2 tests after assuring equality of standard deviations by the Leven’s test; remnant cholesterol difference was analyzed using the Kolmogorov–Smirnov test. Qualitative variables are presented as percentages, and differences were analyzed by ANOVA test. In-hospital mortality predictors were evaluated by binomial logistic regression; the accuracy of the model was tested by its calibration discrimination capacity, evaluated with the area under the curve of the probability predicted by the model, both included in the calibration belt21 that creates a confidence band for the calibration curve based on a function that relates expected to observed probabilities across classes of risk.
Survival analyses were performed after verifying the proportional risk assumption by the Schoenfeld residuals test. The cumulative probability of all-cause mortality and cardiovascular mortality were calculated using the Kaplan–Meier method (a log-rank test was used to compare the survival distributions of two samples). Prior to entry into regression models, remnant cholesterol levels were expanded using fractional polynomials so as not to assume linearity of the effect. Multivariate analyses were performed by Cox regression, and results are presented as hazard ratio (HR) and corresponding 95% confidence intervals (CIs). The incidence of post-discharge HF could be affected by patients’ death, and therefore, the usual techniques for time-to-event analysis would provide biased or un-interpretable results due to the presence of competing risks and the Kaplan–Meier estimation will overestimate the real HF incidence.16,22 With the aim of avoiding such effects, we applied the model introduced by Fine and Gray23 to test the competing events. Thereafter, the incidence of HF was assessed by competing risk regression analyses, taking all-cause mortality as competing event, and results are presented as sub-HR (sHR) and corresponding 95% CI. Harrell’s C-statistic test was used to assess the model’s discrimination; meanwhile, calibration was tested by the Gronnesby and Borgan test. Patients lost during follow-up were categorized as missing, as well as those who lacked any of the main variables for the analyses, although these were very few. Statistical difference was accepted at P < 0.05. All analyses were performed using STATA 14.3 (StataCorp. 2009. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Results
Baseline characteristics
Among the 7479 patients, median (interquartile range) remnant cholesterol level was 28 (21–39) mg/dL, and 3429 (45.85%) patients had levels ≥30 mg/dL. Significantly higher levels of remnant cholesterol were observed in patients with diabetes, current smokers, body mass index (BMI) >30 kg/m2 as well as those without previous cardiovascular disease or premature ACS. No gender differences were observed in remnant cholesterol levels. As shown in Table 1, patients with remnant cholesterol ≥30 mg/dL had lower mean age and higher BMI and especially in the categories of obesity or morbid obesity but, also, higher prevalence of all cardiovascular risk factors, except hypertension. The prevalence of previous cardiac or non-cardiac cardiovascular disease was lower in patients with remnant cholesterol ≥30 mg/dL, and one third were categorized as premature ACS.
| . | Total . | Cholesterol remnants . | . | |
|---|---|---|---|---|
| . | . | <30 mg/dL . | ≥30 mg/dL . | P . |
| n | 7479 | 4050 (54.15) | 3429 (45.85) | |
| Age | 66.66 (12.97) | 69.08 (12.47) | 63.81 (12.96) | <0001 |
| Females | 27.57% | 27.80% | 27.30% | 0626 |
| BMI (kg/m2) | 28.52 (4.18) | 27.94 (4.06) | 29.22 (4.22) | <0001 |
| ȃBMI 25–30 kg/m2 | 18.61% | 22.40% | 14.14% | <0001 |
| ȃBMI 25–29.99 kg/m2 | 53.00% | 54.57% | 51.15% | |
| ȃBMI 30–34.99 kg/m2 | 28.39% | 23.04% | 34.70% | |
| ȃBMI ≥35 kg/m2 | 6.87% | 5.16% | 8.89% | |
| Diabetes | 27.92% | 26.79% | 29.25% | 0018 |
| Hypertension | 57.69% | 59.53% | 55.53% | <0.001 |
| Current smokers | 28.17% | 23.73% | 33.42% | <0.001 |
| Dyslipidaemia | 47.96% | 44.54% | 52.00% | <0.001 |
| Previous CHD | 21.01% | 22.44% | 19.31% | 0001 |
| Previous heart failure | 3.37% | 4.17% | 2.42% | <0.001 |
| Peripheral arterial disease | 7.88% | 7.26% | 8.60% | 0.032 |
| Previous stroke | 6.46% | 7.06% | 5.75% | 0.021 |
| Atrial fibrillation | 11.00% | 13.06% | 8.57% | <0.001 |
| Statin treatment | 28.69% | 30.05% | 27.09% | <0.001 |
| COPD | 9.21% | 10.20% | 8.05% | 0.001 |
| STEMI | 36.45% | 39.09% | 33.33% | <0.001 |
| Premature ACS | 26.33% | 20.15% | 33.62% | <0.001 |
| GRACE score | 142.28 (39.17) | 148.23 (39.63) | 135.26 (37.44) | <0.001 |
| GRACE score >140 | 48.34% | 54.79% | 40.71% | <0.001 |
| LVEF (%) | 54.91 (11.20) | 53.78 (11.73) | 56.24 (10.40) | <0.001 |
| Angiography | 92.71% | 92.72% | 92.71% | 0.991 |
| Revascularization | 77.82% | 79.78% | 75.50% | <0.001 |
| Complete revascularization | 49.55% | 49.78% | 49.29% | 0.671 |
| . | Total . | Cholesterol remnants . | . | |
|---|---|---|---|---|
| . | . | <30 mg/dL . | ≥30 mg/dL . | P . |
| n | 7479 | 4050 (54.15) | 3429 (45.85) | |
| Age | 66.66 (12.97) | 69.08 (12.47) | 63.81 (12.96) | <0001 |
| Females | 27.57% | 27.80% | 27.30% | 0626 |
| BMI (kg/m2) | 28.52 (4.18) | 27.94 (4.06) | 29.22 (4.22) | <0001 |
| ȃBMI 25–30 kg/m2 | 18.61% | 22.40% | 14.14% | <0001 |
| ȃBMI 25–29.99 kg/m2 | 53.00% | 54.57% | 51.15% | |
| ȃBMI 30–34.99 kg/m2 | 28.39% | 23.04% | 34.70% | |
| ȃBMI ≥35 kg/m2 | 6.87% | 5.16% | 8.89% | |
| Diabetes | 27.92% | 26.79% | 29.25% | 0018 |
| Hypertension | 57.69% | 59.53% | 55.53% | <0.001 |
| Current smokers | 28.17% | 23.73% | 33.42% | <0.001 |
| Dyslipidaemia | 47.96% | 44.54% | 52.00% | <0.001 |
| Previous CHD | 21.01% | 22.44% | 19.31% | 0001 |
| Previous heart failure | 3.37% | 4.17% | 2.42% | <0.001 |
| Peripheral arterial disease | 7.88% | 7.26% | 8.60% | 0.032 |
| Previous stroke | 6.46% | 7.06% | 5.75% | 0.021 |
| Atrial fibrillation | 11.00% | 13.06% | 8.57% | <0.001 |
| Statin treatment | 28.69% | 30.05% | 27.09% | <0.001 |
| COPD | 9.21% | 10.20% | 8.05% | 0.001 |
| STEMI | 36.45% | 39.09% | 33.33% | <0.001 |
| Premature ACS | 26.33% | 20.15% | 33.62% | <0.001 |
| GRACE score | 142.28 (39.17) | 148.23 (39.63) | 135.26 (37.44) | <0.001 |
| GRACE score >140 | 48.34% | 54.79% | 40.71% | <0.001 |
| LVEF (%) | 54.91 (11.20) | 53.78 (11.73) | 56.24 (10.40) | <0.001 |
| Angiography | 92.71% | 92.72% | 92.71% | 0.991 |
| Revascularization | 77.82% | 79.78% | 75.50% | <0.001 |
| Complete revascularization | 49.55% | 49.78% | 49.29% | 0.671 |
ACS, acute coronary syndrome; BMI, body mass index; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; LVEF, left ventricle ejection fraction; STEMI, ST-elevation myocardial infarction.
| . | Total . | Cholesterol remnants . | . | |
|---|---|---|---|---|
| . | . | <30 mg/dL . | ≥30 mg/dL . | P . |
| n | 7479 | 4050 (54.15) | 3429 (45.85) | |
| Age | 66.66 (12.97) | 69.08 (12.47) | 63.81 (12.96) | <0001 |
| Females | 27.57% | 27.80% | 27.30% | 0626 |
| BMI (kg/m2) | 28.52 (4.18) | 27.94 (4.06) | 29.22 (4.22) | <0001 |
| ȃBMI 25–30 kg/m2 | 18.61% | 22.40% | 14.14% | <0001 |
| ȃBMI 25–29.99 kg/m2 | 53.00% | 54.57% | 51.15% | |
| ȃBMI 30–34.99 kg/m2 | 28.39% | 23.04% | 34.70% | |
| ȃBMI ≥35 kg/m2 | 6.87% | 5.16% | 8.89% | |
| Diabetes | 27.92% | 26.79% | 29.25% | 0018 |
| Hypertension | 57.69% | 59.53% | 55.53% | <0.001 |
| Current smokers | 28.17% | 23.73% | 33.42% | <0.001 |
| Dyslipidaemia | 47.96% | 44.54% | 52.00% | <0.001 |
| Previous CHD | 21.01% | 22.44% | 19.31% | 0001 |
| Previous heart failure | 3.37% | 4.17% | 2.42% | <0.001 |
| Peripheral arterial disease | 7.88% | 7.26% | 8.60% | 0.032 |
| Previous stroke | 6.46% | 7.06% | 5.75% | 0.021 |
| Atrial fibrillation | 11.00% | 13.06% | 8.57% | <0.001 |
| Statin treatment | 28.69% | 30.05% | 27.09% | <0.001 |
| COPD | 9.21% | 10.20% | 8.05% | 0.001 |
| STEMI | 36.45% | 39.09% | 33.33% | <0.001 |
| Premature ACS | 26.33% | 20.15% | 33.62% | <0.001 |
| GRACE score | 142.28 (39.17) | 148.23 (39.63) | 135.26 (37.44) | <0.001 |
| GRACE score >140 | 48.34% | 54.79% | 40.71% | <0.001 |
| LVEF (%) | 54.91 (11.20) | 53.78 (11.73) | 56.24 (10.40) | <0.001 |
| Angiography | 92.71% | 92.72% | 92.71% | 0.991 |
| Revascularization | 77.82% | 79.78% | 75.50% | <0.001 |
| Complete revascularization | 49.55% | 49.78% | 49.29% | 0.671 |
| . | Total . | Cholesterol remnants . | . | |
|---|---|---|---|---|
| . | . | <30 mg/dL . | ≥30 mg/dL . | P . |
| n | 7479 | 4050 (54.15) | 3429 (45.85) | |
| Age | 66.66 (12.97) | 69.08 (12.47) | 63.81 (12.96) | <0001 |
| Females | 27.57% | 27.80% | 27.30% | 0626 |
| BMI (kg/m2) | 28.52 (4.18) | 27.94 (4.06) | 29.22 (4.22) | <0001 |
| ȃBMI 25–30 kg/m2 | 18.61% | 22.40% | 14.14% | <0001 |
| ȃBMI 25–29.99 kg/m2 | 53.00% | 54.57% | 51.15% | |
| ȃBMI 30–34.99 kg/m2 | 28.39% | 23.04% | 34.70% | |
| ȃBMI ≥35 kg/m2 | 6.87% | 5.16% | 8.89% | |
| Diabetes | 27.92% | 26.79% | 29.25% | 0018 |
| Hypertension | 57.69% | 59.53% | 55.53% | <0.001 |
| Current smokers | 28.17% | 23.73% | 33.42% | <0.001 |
| Dyslipidaemia | 47.96% | 44.54% | 52.00% | <0.001 |
| Previous CHD | 21.01% | 22.44% | 19.31% | 0001 |
| Previous heart failure | 3.37% | 4.17% | 2.42% | <0.001 |
| Peripheral arterial disease | 7.88% | 7.26% | 8.60% | 0.032 |
| Previous stroke | 6.46% | 7.06% | 5.75% | 0.021 |
| Atrial fibrillation | 11.00% | 13.06% | 8.57% | <0.001 |
| Statin treatment | 28.69% | 30.05% | 27.09% | <0.001 |
| COPD | 9.21% | 10.20% | 8.05% | 0.001 |
| STEMI | 36.45% | 39.09% | 33.33% | <0.001 |
| Premature ACS | 26.33% | 20.15% | 33.62% | <0.001 |
| GRACE score | 142.28 (39.17) | 148.23 (39.63) | 135.26 (37.44) | <0.001 |
| GRACE score >140 | 48.34% | 54.79% | 40.71% | <0.001 |
| LVEF (%) | 54.91 (11.20) | 53.78 (11.73) | 56.24 (10.40) | <0.001 |
| Angiography | 92.71% | 92.72% | 92.71% | 0.991 |
| Revascularization | 77.82% | 79.78% | 75.50% | <0.001 |
| Complete revascularization | 49.55% | 49.78% | 49.29% | 0.671 |
ACS, acute coronary syndrome; BMI, body mass index; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; LVEF, left ventricle ejection fraction; STEMI, ST-elevation myocardial infarction.
As expected, patients with elevated remnant cholesterol had a less favourable lipid profile, as reflected by higher TG/HDL ratio, TGGi, and non-HDL cholesterol (Table 2). No differences in haemoglobin or kidney function were observed. Age (r: −0.29) and BMI (r: 0.44) were the variables more strongly correlated to cholesterol remnant (Figure 1). At any given age, the risk of having remnant cholesterol ≥30 was higher as BMI increased (Figure 2).
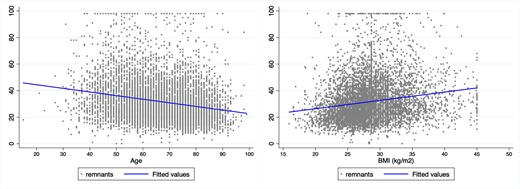
Correlation between remnant cholesterol and age or body mass index.
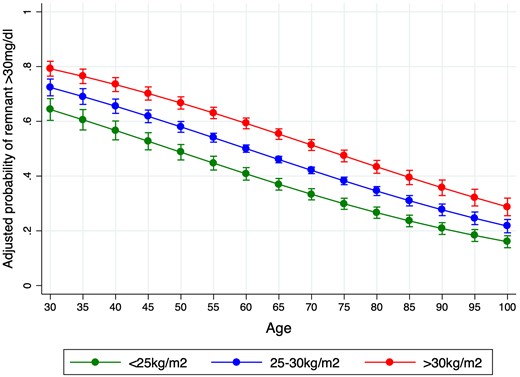
Adjusted probability of remnant cholesterol >30 mg/dL according to age and the presence of overweight (body mass index 25–30 kg/m2) or obesity (body mass index >30 kg/m2).
| . | Total . | Cholesterol remnants . | . | |
|---|---|---|---|---|
| . | . | <30 mg/dL . | ≥30 mg/dL . | P . |
| Number | 7479 | 4050 (54.15%) | 3429 (45.85%) | |
| Cholesterol remnants (mg/dL) | 28 (21–39) | 21 (17–25) | 40 (34–50) | <0.001 |
| Haemoglobin (g/dL) | 13.88 (2.90) | 13.65 (2.97) | 14.14 (2.80) | <0.001 |
| Creatinine (mg/dL) | 1.07 (1.07) | 1.05 (0.49) | 1.09 (1.48) | 0.101 |
| GFR mL/min/1.72 m2 | 74.93 (23.60) | 73.81 (22.78) | 76.25 (24.48) | <0.001 |
| GFR <60 mL/min/1.72 m2 | 25.64% | 26.62% | 24.48% | 0.035 |
| Fasting glucose (mg/dL) | 146.54 (122.12) | 142.53 (147.12) | 151.29 (83.23) | 0.002 |
| HbA1c (%) | 6.26 (1.51) | 6.16 (1.34) | 6.40 (1.69) | <0.001 |
| Total cholesterol (mg/dL) | 175.82 (44.67) | 161.52 (39.78) | 192.71 (44.23) | <0.001 |
| LDLc (mg/dL) | 107.08 (38.22) | 100.70 (35.52) | 114.62 (39.88) | <0.001 |
| HDLc (mg/dL) | 36.65 (12.56) | 39.88 (12.85) | 32.83 (11.04) | <0.001 |
| Triglycerides (mg/dL) | 158.71 (79.60) | 104.82 (0.41) | 222.36 (74.38) | <0.001 |
| Triglycerides/HDL ratio | 5.12 (4.01) | 2.96 (1.39) | 7.69 (4.56) | <0.001 |
| Triglycerides-glucose index | 9.14 (0.66) | 8.77 (0.51) | 9.58 (8.55) | <0.001 |
| Non-HDL cholesterol | 139.23 (44.83) | 121.62 (36.88) | 160.03 (44.48) | <0.001 |
| . | Total . | Cholesterol remnants . | . | |
|---|---|---|---|---|
| . | . | <30 mg/dL . | ≥30 mg/dL . | P . |
| Number | 7479 | 4050 (54.15%) | 3429 (45.85%) | |
| Cholesterol remnants (mg/dL) | 28 (21–39) | 21 (17–25) | 40 (34–50) | <0.001 |
| Haemoglobin (g/dL) | 13.88 (2.90) | 13.65 (2.97) | 14.14 (2.80) | <0.001 |
| Creatinine (mg/dL) | 1.07 (1.07) | 1.05 (0.49) | 1.09 (1.48) | 0.101 |
| GFR mL/min/1.72 m2 | 74.93 (23.60) | 73.81 (22.78) | 76.25 (24.48) | <0.001 |
| GFR <60 mL/min/1.72 m2 | 25.64% | 26.62% | 24.48% | 0.035 |
| Fasting glucose (mg/dL) | 146.54 (122.12) | 142.53 (147.12) | 151.29 (83.23) | 0.002 |
| HbA1c (%) | 6.26 (1.51) | 6.16 (1.34) | 6.40 (1.69) | <0.001 |
| Total cholesterol (mg/dL) | 175.82 (44.67) | 161.52 (39.78) | 192.71 (44.23) | <0.001 |
| LDLc (mg/dL) | 107.08 (38.22) | 100.70 (35.52) | 114.62 (39.88) | <0.001 |
| HDLc (mg/dL) | 36.65 (12.56) | 39.88 (12.85) | 32.83 (11.04) | <0.001 |
| Triglycerides (mg/dL) | 158.71 (79.60) | 104.82 (0.41) | 222.36 (74.38) | <0.001 |
| Triglycerides/HDL ratio | 5.12 (4.01) | 2.96 (1.39) | 7.69 (4.56) | <0.001 |
| Triglycerides-glucose index | 9.14 (0.66) | 8.77 (0.51) | 9.58 (8.55) | <0.001 |
| Non-HDL cholesterol | 139.23 (44.83) | 121.62 (36.88) | 160.03 (44.48) | <0.001 |
GFR, glomerular filtration rate; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol.
| . | Total . | Cholesterol remnants . | . | |
|---|---|---|---|---|
| . | . | <30 mg/dL . | ≥30 mg/dL . | P . |
| Number | 7479 | 4050 (54.15%) | 3429 (45.85%) | |
| Cholesterol remnants (mg/dL) | 28 (21–39) | 21 (17–25) | 40 (34–50) | <0.001 |
| Haemoglobin (g/dL) | 13.88 (2.90) | 13.65 (2.97) | 14.14 (2.80) | <0.001 |
| Creatinine (mg/dL) | 1.07 (1.07) | 1.05 (0.49) | 1.09 (1.48) | 0.101 |
| GFR mL/min/1.72 m2 | 74.93 (23.60) | 73.81 (22.78) | 76.25 (24.48) | <0.001 |
| GFR <60 mL/min/1.72 m2 | 25.64% | 26.62% | 24.48% | 0.035 |
| Fasting glucose (mg/dL) | 146.54 (122.12) | 142.53 (147.12) | 151.29 (83.23) | 0.002 |
| HbA1c (%) | 6.26 (1.51) | 6.16 (1.34) | 6.40 (1.69) | <0.001 |
| Total cholesterol (mg/dL) | 175.82 (44.67) | 161.52 (39.78) | 192.71 (44.23) | <0.001 |
| LDLc (mg/dL) | 107.08 (38.22) | 100.70 (35.52) | 114.62 (39.88) | <0.001 |
| HDLc (mg/dL) | 36.65 (12.56) | 39.88 (12.85) | 32.83 (11.04) | <0.001 |
| Triglycerides (mg/dL) | 158.71 (79.60) | 104.82 (0.41) | 222.36 (74.38) | <0.001 |
| Triglycerides/HDL ratio | 5.12 (4.01) | 2.96 (1.39) | 7.69 (4.56) | <0.001 |
| Triglycerides-glucose index | 9.14 (0.66) | 8.77 (0.51) | 9.58 (8.55) | <0.001 |
| Non-HDL cholesterol | 139.23 (44.83) | 121.62 (36.88) | 160.03 (44.48) | <0.001 |
| . | Total . | Cholesterol remnants . | . | |
|---|---|---|---|---|
| . | . | <30 mg/dL . | ≥30 mg/dL . | P . |
| Number | 7479 | 4050 (54.15%) | 3429 (45.85%) | |
| Cholesterol remnants (mg/dL) | 28 (21–39) | 21 (17–25) | 40 (34–50) | <0.001 |
| Haemoglobin (g/dL) | 13.88 (2.90) | 13.65 (2.97) | 14.14 (2.80) | <0.001 |
| Creatinine (mg/dL) | 1.07 (1.07) | 1.05 (0.49) | 1.09 (1.48) | 0.101 |
| GFR mL/min/1.72 m2 | 74.93 (23.60) | 73.81 (22.78) | 76.25 (24.48) | <0.001 |
| GFR <60 mL/min/1.72 m2 | 25.64% | 26.62% | 24.48% | 0.035 |
| Fasting glucose (mg/dL) | 146.54 (122.12) | 142.53 (147.12) | 151.29 (83.23) | 0.002 |
| HbA1c (%) | 6.26 (1.51) | 6.16 (1.34) | 6.40 (1.69) | <0.001 |
| Total cholesterol (mg/dL) | 175.82 (44.67) | 161.52 (39.78) | 192.71 (44.23) | <0.001 |
| LDLc (mg/dL) | 107.08 (38.22) | 100.70 (35.52) | 114.62 (39.88) | <0.001 |
| HDLc (mg/dL) | 36.65 (12.56) | 39.88 (12.85) | 32.83 (11.04) | <0.001 |
| Triglycerides (mg/dL) | 158.71 (79.60) | 104.82 (0.41) | 222.36 (74.38) | <0.001 |
| Triglycerides/HDL ratio | 5.12 (4.01) | 2.96 (1.39) | 7.69 (4.56) | <0.001 |
| Triglycerides-glucose index | 9.14 (0.66) | 8.77 (0.51) | 9.58 (8.55) | <0.001 |
| Non-HDL cholesterol | 139.23 (44.83) | 121.62 (36.88) | 160.03 (44.48) | <0.001 |
GFR, glomerular filtration rate; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol.
In-hospital mortality
In-hospital mortality was 3.75% (280 patients). After adjustment by age, gender, previous cardiovascular disease, and GRACE score, remnant cholesterol was not associated to higher mortality risk (odds ratio: 0.89; 95% CI 0.64–1.10; P = 0.21) (Figure 3).
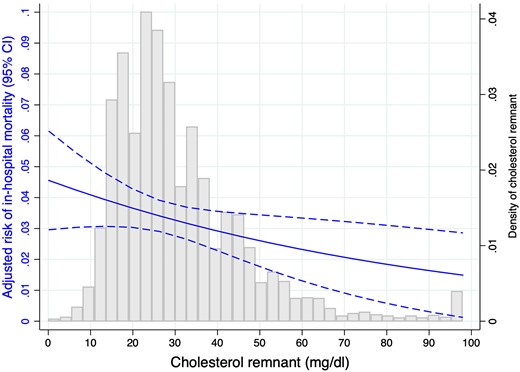
Adjusted risk of in-hospital mortality according to remnant cholesterol and histogram for remnant cholesterol distribution.
Post-discharge prognosis
Follow-up was available for 93% of the patients with a median follow-up of 57 months (interquartile range 22–79). All-cause mortality was 20.86% (1741 patients), 14.2% were due to cardiovascular causes, and 1078 patients (12.92%) had a first re-admission for HF. Multivariate analysis, adjusted by age, sex, hypertension, diabetes, GRACE score, and medical treatments at discharge, verified the independent and linear risk of all-cause mortality (P = 0.028) and HF (P = 0.004) associated to cholesterol remnant levels (Figure 4). After categorization of remnant cholesterol, we found that levels >60 mg/dL were associated to higher risk of mortality (HR: 1.49 95% CI 1.08–2.06; P = 0.016), cardiovascular mortality (HR: 1.49 95% CI 1.08–2.06; P = 0.016), and HF re-admission (sHR: 1.55 95% CI 1.14–2.11; P = 0.005).

Adjusted risk of all-cause mortality, cardiovascular mortality, or heart failure according to baseline remnant cholesterol levels.
Remnant cholesterol compared to other parameters of lipid-residual risk
Neither TG/HDL ratio, TC/HDL ratio, TGGi, nor non-HDL cholesterol was associated to higher in-hospital mortality risk. In contrast, TGGi was associated to higher long-term risk of mortality (HR: 1.16 95% CI 1.06–1.27; P < 0.01) and HF (sHR: 1.14 95% CI 1.02–1.27; P = 0.025).
Discussion
The analysis of a large cohort of patients admitted for ACS underlines that almost half of them had remnant cholesterol ≥30 mg/dL. Remnant cholesterol levels were not associated to higher risk of in-hospital mortality, but they were linearly and significantly associated to higher risk mortality and HF re-admission during the follow-up. Since clinical characteristics and event rate were similar to previous reports,3–7,24,25 we believe that our results might be representative and clinically relevant.
Remnant cholesterol are TG-risk lipoproteins that are mostly unable to enter the artery wall but contribute to atherosclerosis initiation due to their high cholesterol content per particle but, also, higher promotion of cholesterol accumulation by macrophages than LDL particles.2 Our study highlights that nearly half of the patients admitted for an ACS have elevated remnant cholesterol >30 mg/dL, and it is clearly related to higher BMI and younger age. Premature cardiovascular disease is usually linked to genetic and severe metabolic disorders, and our results demonstrated the high burden of remnant cholesterol and the rest of the so-called lipid-residual risk in young patients admitted for an ACS.26 STEMI represented two thirds of the cases, and patients admitted for premature ACS had more frequently STEMI (42.71% vs. 32.82%; P < 0.01), higher remnant cholesterol levels [36.41 (17.80) vs. 30.06 (14.86) mg/dL; P < 0.001], and remnant cholesterol >30 mg/dL (58.56% vs. 41.31%; P < 0.01). Nonetheless, patients with elevated remnant cholesterol presented less frequently as STEMI despite lower mean age; these findings could suggest that remnant cholesterol might be related to less abrupt forms of atherosclerosis and thrombosis2 but at younger ages. Interestingly, cholesterol remnant levels were significantly lower at higher ages what might underlie the multifactorial mechanisms implied in its metabolism; nonetheless, remnant cholesterol was higher in patients with overweight or obesity at any given age. We believe this demarks the key role of obesity on cholesterol remnants.
Remnant cholesterol levels had no implication on in-hospital mortality but were independently associated to higher long-term risk of death or HF. These results are very relevant and warrant future investigations. In-hospital outcomes of ACS patients are strongly related to the clinical presentation (Killip class, hypotension, myocardial damage, or ST segment elevation), age, and other well-established risk factors (renal function, revascularization or haemoglobin).12,27 Remnant cholesterol levels reflect previous metabolic status of each patient rather than acute changes. Cholesterol metabolism and alterations are clearly related to all the atherosclerosis process but might not be related to the clinical presentation of the ACS and, thus, might not have an impact on in-hospital outcomes. In contrast, we found a linear and independent relationship between remnant cholesterol levels and mortality what reflects their long-term effect of secondary prevention. Moreover, we found an increased risk for values >60 mg/dL (≥1.6 mmol/L), as previously reported by the Copenhagen General Population Study.6 Nonetheless, we found that the TGGi conferred higher risk of long-term mortality or HF what is in concordance with the results reported by Atherosclerosis Risk in Communities study.28 The assessment of cholesterol remnant is much easier that TGGi and maybe both parameters should be considered in the assessment of patients’ cardiovascular risk.
The so-called lipid residual risk is a concept derived for the sustained incidence of major cardiovascular event despite LDLc control. Patients with established cardiovascular disease and/or severe hypercholesterolemia usually have small and dense LDL particles.29 Particle’s size can be measured by magnetic resonance or other complex methods but the TG/HDL has demonstrated to correlate quite fairly.30 In fact, TG/HDL has been proposed as the leading risk factor for a first myocardial infarction in young patients.11 Similarly, low HDLc levels were associated to higher risk of ischaemic events in a large sample of patients recruited from primary care in Spain9 or in patients with myocardial infarction.31 The comparison patients admitted for myocardial infarction vs. non-ischaemic chest pain in our institution demonstrated that low HDLc, rather than LDLc, was the variable with highest association to a final diagnosis of myocardial infarction.8 A pooled analysis of 10 trials and 5754 patients demonstrated a high variability in plaques regression despite effective changes in LDLc.4 More clinically, a sub-analysis of the PREDIMED trial demonstrated that remnant cholesterol >30 mg/dL was the leading risk factor for the incidence of the first major cardiovascular event in patients at high cardiovascular risk.3 In contrast, 106 937 individuals from the Copenhagen General Population Study25 found an increased long-term risk of peripheral disease and myocardial infarction in patients with levels 58 mg/dL (≥1.5 mmol/L), what is very similar to our results and reinforces our conclusions. Two analyses of the Atherosclerosis Risk in Communities study have demonstrated the independent role of remnant cholesterol on ischaemic events7 and baseline TGGi index on HF incidence.28 In all these studies, as we also did, remnant cholesterol was not measured directly, and although some genetic variants could influence lipoprotein parameters,32 the clinical impacts of the results are all pretty well balanced. Thereafter, we believe that our results are in good concordance with recent evidence that supports the role of remnant cholesterol and lipid-residual risk on cardiovascular disease incidence and prognosis.
Thereafter, many lipid variables are actively involved in the atherosclerotic process beyond the effect of LDLc. Subsequently, the global assessment of the lipid profile might have relevant role in primary and secondary prevention of cardiovascular disease. In this line, recent results have demonstrated the significant effect of proprotein convertase subtilisin/kexin type 9 inhibitors on cholesterol remnants, TG/HDL and TC/HDL ratios as well as TGGi that might provide a wider view of the beneficial effects of this lipid-lowering therapy.33
Finally, we also found a linear risk of HF associated to remnant cholesterol. Coronary heart disease is the leading risk factor of HF, and new onset of HF in patients discharge after an ACS is high.16,34 The independent effect of remnant cholesterol on HF in this population might be related to higher burden of atherosclerosis, more associated metabolic disorders, or other unknown mechanism that warrant future studies.2 Myocardial metabolism adaptation is critically involved in HF incidence after a myocardial infarction,35 and inhibition of sodium-glucose cotransporter-2 has demonstrated to improve outcomes in patients with HF, regardless of the presence of diabetes or left ventricle ejection fraction.36 LDLc reduction is one of the primary objectives in secondary prevention of ACS patients, but our results suggest that assessment of remnant cholesterol might demark, also, patients at higher long-term risk of mortality or HF.
Our study has some limitations. First, some inherent limitations to the study design are being an observational and retrospective study, the possible long-term variations in medical treatments, or other uncontrolled variables. Second, remnant cholesterol was not measured directly, and although it might be influenced by other lipoprotein parameters,32 the equation used has demonstrated to provide reliable values that have independent prognostic value).3,5,6 Third, 7% of the patients were no available follow-up, and this could have had some impact on the analyses. Since clinical features and long-term event incidence are similar to previous reports,3–7,11,24,25 we believe that the above-presented limitations might not had a relevant impact on our results.
In conclusion, our large cohort of patients admitted for ACS suggests that remnant cholesterol levels have an independent role on long-term prognosis and warrant future studies and trials directed to assess OMTs for patients with elevated remnant cholesterol.
Funding
This study received the support of the Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV) the Spanish National Network for Biomedical Investigation on Cardiovascular Disease.
Data availability
Data available on request.
Author contribution
A.C., B.A.A., D.E., and B.C.-A. designed the study and drafted the manuscript. J.M.G.-A., M.R.-M., M.A.Q., and R.A.-B. contributed to the acquisition. P.Z. and J.R.G.-J. critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
References
Author notes
Conflict of interest: A.C. reports (i) honoraria for lectures from AstraZeneca, AMGEN, Bristol-Myers Squibb, Ferrer, Boehringer-Ingelheim, MSD, Daichy Sankio, Novartis, Novo Nordisk, Sanofi and Amarin; (ii) consulting fees from AstraZeneca, Ferrer, Sanofi, AMGEN, Novartis, Lilly, Novo Nordisk and Amarin. J.R.G.-J. reports (i) honoraria for lectures from Eli Lilly Co, Daiichi Sankyo, Inc., Bayer, Pfizer, Abbott, Boehringer-Ingelheim, MSD, Ferrer, and Bristol-Myers Squibb; (ii) consulting fees from AstraZeneca, Ferrer, Bayer, Boehringer-Ingelheim; (iii) research grants from AstraZeneca, Boehringer-Ingelheim and Daichii-Sankyo.




Comments