-
PDF
- Split View
-
Views
-
Cite
Cite
Kai Jin, Does coronary microvascular dysfunction have a role in cardiovascular oncology?, European Journal of Preventive Cardiology, Volume 30, Issue 3, February 2023, Pages 206–208, https://doi.org/10.1093/eurjpc/zwac229
Close - Share Icon Share
This editorial refers to ‘Coronary microvascular dysfunction is an independent predictor of developing cancer in patients with non-obstructive coronary artery disease’, by N. Rajai et al. https://doi.org/10.1093/eurjpc/zwac184.
Cardiovascular disease (CVD) and cancer are the two leading causes of death worldwide.1 Substantial evidence showed that cancer patients were at increased risk of developing fatal and non-fatal cardiovascular events.2–4 The overall risk for CVD in cancer patients is two to six times higher than that in the general population.4 The mechanisms of increased CVD risk in cancer patients may include cancer-associated proinflammatory and hypercoagulability, as well as cancer therapy-induced cardiotoxicity with complex mechanisms related to the type of drugs and treatment.2,5 Traditional cardio-oncology has focused on cardiovascular risk in patients with cancer treatment. Growing evidence shows that the patients with CVD are at an increased risk of developing cancer than the general population, suggesting a possible bidirectional link between CVD and cancer, the phenomenon referred to ‘reverse cardio-oncology’.6–8 Given that both diseases share common risk factors (i.e. ageing, hypertension, diabetes) and pathophysiological pathways (i.e. inflammation, hypoxia), there is gaining interest in exploring the complex relationships between CVD and cancer, for management and prevention of these two diseases.9–11
Coronary microvascular dysfunction (CMD), a subset of disorders affecting the structure and/or function of the coronary microcirculation, is emerging entity in the evaluation of patients with suspected ischaemic myocardial syndromes in the absence of obstructive coronary artery disease (CAD).12 Coronary microvascular dysfunction is characterized by impaired microvascular vasodilation in response to an inadequate increase in blood flow to match myocardial oxygen resulting in reduced coronary flow reserve (CFR).12 Coronary flow reserve is a gold standard to assess CMD in patients without significant obstructive CAD,13 which can be measured invasively as an adjunct to coronary angiography or non-invasively, such as using positron emission tomography (PET).14 Compelling evidence show that CMD is associated with an increased risk of major adverse cardiac events (MACE) including myocardial infarction, stroke, and sudden death in an array of CVD including ischaemic heart disease, heart failure, and Type 2 diabetes.12,15 Besides its role in evaluating cardiovascular risk in patients with CVD, emerging evidence showed that microvascular dysfunction is associated with future risk of cancer and increased cardiovascular risk in cancer patients.16–18
In this issue of the EJPC, Rajai and colleagues19 explored the role of CMD in relation to cancer in a cohort of patients with angina and non-obstructive coronary artery disease (NOCAD) using invasively measuredly CFR. Specifically, they assessed the (i) association between CMD and the future risk of developing solid tumour and (ii) association between CMD and MACE in NOCAD patients with a history of cancer. A total of 1042 patients with NOCAD who attended the Mayo Clinic between 2000 and 2019 for the assessment of chest pain were included in the analysis. The presence of CMD was defined as invasive CFR ≤2.5 based on their previous studies. The outcomes of interest were the incident cancer and MACE. At baseline, patients with CMD had worse CV risk profiles than non-CMD patients. Patients with CMD were older, female, and more likely to have diabetes, hyperlipidaemia, cancer history, and poor renal function. During a median of 9 years follow-up, in NOCAD patients who were free of cancer at study entry (n = 917), a total of 141 patients (15.5%) were found to have a cancer diagnosis. After adjusting for common CVD risk factors including age, sex, BMI, smoking, diabetes, hypertension, and renal function, the presence of CMD was associated with increased risk of cancer [hazard ratio (HR) 1.45, 95% confidence interval (CI) 1.02–2.09, P = 0.04] (Figure 1). When examining the outcome of MACE, CMD was associated with a greater risk of MACE in patients with cancer history [odds ratios (OR) 2.50, 95% CI 1.003–6.20, P = 0.04] (Figure 2) than those without (OR 1.4, 95% CI 1.01–1.90, P = 0.04) after adjustment of common vascular risk factors.
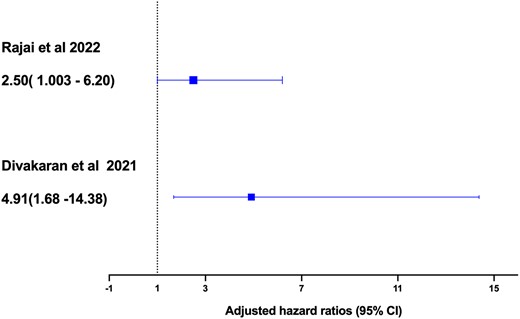
Association between coronary microvascular dysfunction and major adverse cardiac events by comparing the current study by Rajai et al.19 with the previous study by Divakaran et al.16 In Rajai et al.,19 coronary microvascular dysfunction was defined as invasive coronary flow reserve ≤2.5. In Toya et al.,18 microvascular dysfunction was defined by reactive hyperaemia peripheral arterial tonometry index≤2.0. In Divakaran et al.,16 coronary microvascular dysfunction was measured by myocardial flow reserve using positron emission tomography. Rajai et al : Adjusted odds risk (OR)
These findings provide new evidence that the presence of CMD may have a prognostic role to evaluate the future risk of cancer in patients with NOCAD. The observed relation between CMD and incident cancer is less likely explained by reverse causality because authors used landmark time of 1, 3, and 5 years and showed patients with CMD were significantly likely to develop cancer at all three time points. Results of the present study support the findings from the previous study by the same group of authors using the reactive hyperaemia peripheral arterial tonometry (RH-PAT) index to assess microvascular dysfunction in relation to cancer risk.18 In this study, Toya et al.18 showed the presence of microvascular dysfunction defined as RH-PAT index≤2.0 was associated with increased risk of incident solid tumour in patients with chest pain and/or cardiovascular risk (HR 2.79, 95% CI 1.24–6.41; Figure 1). Together, these findings may support the shared pathophysiologic pathways including chronic inflammation, excess oxidative, and hypoxia for both CVD and cancer.20,21 Impaired microcirculation may contribute to the creation of a pro-oncogenic environment by potentially activating the angiogenetic pathway and promote tumour growth.18 Inflammatory markers including C-reactive protein (CRP) and interleukin (IL)-6 are associated with CAD and in malignant process like metastasis, angiogenesis, immune evasion, and cancer cell invasion.11,22 Indeed, in patients with stable CVD, chronic systemic low-grade inflammation, measured by CRP, is a risk factor for cancer, in particular lung cancer.10 The results from Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) further support this concept by showing lowering CRP with interleukin-1β antibody can improve cardiovascular outcomes and reduce the cancer incidence and mortality.23
Another important finding is that the presence of CMD in NOCAD patients with the history of cancer was associated with a greater risk of MACE than those without cancer (OR 2.5 vs. OR 1.4). These findings correspond to a previous study by Divakaran et al.16 that assessed the impact of impaired microcirculation on cardiovascular risk in cancer patients. In this study, the authors examined the association between CMD measured by myocardial flow reserve (MFR) using PET and adverse cardiovascular events in a cohort of 87 breast cancer patients with no clinically overt CAD.16 After adjusting common vascular risk factors, family history of CAD, and chronic kidney disease, those in the lowest MFR tertile (<1.7) had a higher incidence of MACE during a median follow-up of 7 years compared with patients in the highest MFR tertile (>2.24) (HR 4.91, 95% CI 1.68–14.38, P = 0.04, Figure 2). These results demonstrated a consistent risk of MACE associated with CMD across different diagnostic modalities in cancer patients and are independent of known vascular risk factors.
The exact mechanisms that contribute to impaired CFR and increased risk of MACE in cancer patients remain unclear and are likely multifactorial. Cancer-associated proinflammatory and hypercoagulability can increase the risk of thromboembolic events including stroke.2 It may also result from the interplay between cancer therapy-induced microcirculation injury including radiation or chemotherapy such as doxorubicin and chronic inflammation that led to myocardial ischaemia, resulting in increased cardiovascular risk in cancer patients.17,24 This is supported by a recent study that showed increased mean cardiac radiation dose was strongly correlated to the decreased global MFR using PET in a cohort of cancer patients following radiotherapy.17 However, the lack of details on the cancer treatment regime in this study limits further interpretation.
The main strength of this study is CMD was confirmed by gold standard invasive measurement, which provides enhanced accuracy of diagnosis compared with non-invasive diagnostic modalities to understand the microvascular function and its relation to cancer risk. The other strengths include long follow-up and taking into account of reverse causality by performing landmark analysis. However, when interpreting these results, the main consideration is that this is a retrospective analysis in a single centre, in which their original study design has not been powered to the cancer outcomes. The small sample size may have an impact on the statistical power to detect true effects. Because cancer is a general term that refers to large groups of heterogeneous diseases, validation of this relationship in future studies with detailed cancer phenotyping is required. Although this analysis adjusted the common risk factors shared by both CVD and cancer, other important interactions between CVD and cancer such as inflammatory markers, cancer therapy, and cardioprotective medication were not included, which require a more careful design before the conclusion can be drawn.
Taken together, the findings of this study advance our current understanding of the complex relationship between CVD and cancer. The results provide new evidence that the presence of CMD defined by invasively measured CRF may have a role to evaluate cancer occurrence in patients with NOCAD, supporting the need for further studies to better understand the cancer risk in patients with CVD. Among patients with a history of cancer, assessment of CMD could potentially have a role in the early detection of cancer treatment-related cardiovascular complications, which help to identify the high-risk group to guide diagnosis and preventive therapies.
Funding
K.J. is funded by Cancer Research UK programme grant (C7923/A29018).
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Journal of Preventive Cardiology or of the European Society of Cardiology.
Conflict of interest: None declared.





Comments