-
PDF
- Split View
-
Views
-
Cite
Cite
Yuta Suzuki, Hidehiro Kaneko, Yuichiro Yano, Akira Okada, Hidetaka Itoh, Kensuke Ueno, Satoshi Matsuoka, Katsuhito Fujiu, Nobuaki Michihata, Taisuke Jo, Norifumi Takeda, Hiroyuki Morita, Isao Yokota, Koichi Node, Hideo Yasunaga, Issei Komuro, Dose-dependent relationship of blood pressure and glycaemic status with risk of aortic dissection and aneurysm, European Journal of Preventive Cardiology, Volume 29, Issue 18, December 2022, Pages 2338–2346, https://doi.org/10.1093/eurjpc/zwac205
Close - Share Icon Share
Abstract
Data on the dose-dependent association of blood pressure (BP) and fasting plasma glucose (FPG) level with the risk of aortic dissection (AD) and aortic aneurysm (AA) are limited.
This observational cohort study included 3 358 293 individuals registered in a health checkup and claims database in Japan [median age, 43 (36–51) years; 57.2% men]. Individuals using BP- or glucose-lowering medications or those with a history of cardiovascular disease were excluded. In a mean follow-up period of 1 199 ± 950 days, 1 095 and 2 177 cases of AD and AA, respectively, were recorded. Compared with normal/elevated BP, hazard ratios (HRs) of Stage 1 and Stage 2 hypertension were 1.89 [95% confidence interval (CI): 1.60–2.22] and 5.87 (95% CI: 5.03–6.84) for AD and 1.37 (95% CI: 1.23–1.52) and 2.17 (95% CI: 1.95–2.42) for AA, respectively. Compared with normal FPG level, HRs of prediabetes and diabetes were 0.82 (95% CI: 0.71–0.94) and 0.48 (95% CI: 0.33–0.71) for AD and 0.94 (95% CI: 0.85–1.03) and 0.61 (95% CI: 0.47–0.79) for AA, respectively. The cubic spline demonstrated that the risk of AD and AA increased with increasing BP but decreased with increasing FPG level. Contour plots using generalized additive models showed that higher systolic BP and lower FPG level were associated with an elevated risk of AD and AA.
Our analysis showed a dose-dependent increase in the risk of AD or AA associated with BP and a similar decrease associated with FPG, and also suggested a potential interaction between hypertension and hyperglycaemia in the development of AD and AA.
See the editorial comment for this article ‘Hypertension and diabetes versus the risk of aortic disease: a new look on prevention?’, by M. Hibino and C.A. Nienaber, https://doi.org/10.1093/eurjpc/zwac259.
Introduction
Aortic dissection (AD) and aortic aneurysm (AA) are life-threatening cardiovascular diseases (CVDs).1–5 Despite advances in treatment strategies, the mortality of acute AD remains high. An analysis of the International Registry of Acute Aortic Dissection reported that the in-hospital mortality of Type A AD was 22%; surgical mortality was 18%.3 Most AAs were asymptomatic, and AAs dilate slowly but progressively. Once AA ruptures, it leads to catastrophic outcomes with extremely high mortality. For example, the in-hospital mortality of ruptured abdominal AA was >50% in the USA (53.1%) and England (65.9%).6 Given such dismal clinical outcomes of AD and AA, preventing these conditions is essential. Therefore, risk stratification for AD and AA is the initial required step in this direction. Similar to other CVDs, hypertension is a major risk factor for both AD and AA.7–10 In contrast, diabetes, which is also a strong risk factor for CVD, has been associated with a lower incidence of AD or AA.11–14 However, because of the relatively low incidence of both AD and AA, there have been fewer epidemiological data on risk factors for these conditions compared to other CVDs, such as ischaemic heart disease and stroke. In addition, the aforementioned epidemiological studies regarding the risk factors of AD or AA have several limitations. First, in most previous studies on AD or AA, analyses using specific values of blood pressure (BP) or fasting plasma glucose (FPG) level have not been conducted; therefore, the dose-dependent relationship is unclear. Second, although a recent study clearly reported a potential dose-response association of BP and incident AD, 7 this study and previous studies included individuals with a history of BP- or glucose-lowering medications, which could have affected the results. Finally, although hypertension and diabetes frequently coexist, the risk of AD or AA when both hypertension and diabetes coexist, remains unclear. Previous epidemiological studies demonstrated that hypertension is positively associated with a risk for AD or AA, but diabetes is inversely associated with a risk for AD or AA, and it is unclear how they affect each other. Hence, this study aimed to analyze a large-scale health checkup and claims database to clarify the association among hypertension, hyperglycaemia and the risk of AD and AA.
Methods
The JMDC Claims Database, which was used in this study, is available for purchase from the JMDC Inc. (https://www.jmdc.co.jp/en/). The JMDC is a healthcare venture company in Japan.
Study population
This was a retrospective observational cohort study that used the JMDC Claims Database (JMDC Inc., Tokyo, Japan), which is a health checkup and administrative claims database in Japan (https://www.jmdc.co.jp/en/jmdc-claims-database/). Most individuals registered in this database are employees working for relatively large companies in Japan under the coverage of ‘kempo’, a health insurance system for employees. This database includes records of individuals’ annual health checkup data [e.g. BP, FPG, body mass index (BMI), self-reported questionnaires regarding lifestyles] and administrative claims data recorded using the International Classification of Diseases, 10th Revision (ICD-10) coding. The JMDC Claims Database has collected the claims data on outpatient care and hospital care in the period of insurance coverage. We extracted the records of individuals enrolled in the JMDC Claims Database between January 2005 and April 2021. Disease status was extracted based on ICD-10 coding. Among the 4 534 334 individuals with available health checkup data, including BP and FPG data, we excluded individuals who met the following criteria: (i) age <20 years (n = 6 868); (ii) history of CVD, including myocardial infarction, angina pectoris, and stroke (n = 140 254); (iii) history of renal disease or dialysis (n = 1 685); (iv) history of AD or AA (n = 2 972); (v) history of Marfan syndrome or Ehlers-Danlos syndrome (n = 127); (vi) use of BP- or glucose-lowering medications (n = 430 011); (vii) missing data on cigarette smoking (n = 262 910); and (viii) missing data on alcohol consumption (n = 331 214). The study population included 3 358 293 participants (see Supplementary material online, Figure S1).
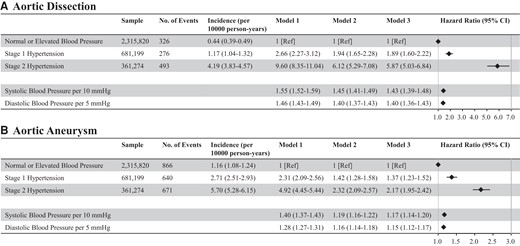
Blood pressure and risk of aortic dissection or aneurysm. The association of blood pressure category with the risk of aortic dissection (A) and aortic aneurysm (B) is shown. We conducted Cox regression analyses. Model 1 included blood pressure category alone (unadjusted model). Model 2 included blood pressure category, age, and sex, and multivariable Cox regression analyses (forced entry model) were conducted. Furthermore, as in Model 3, we added body mass index, fasting plasma glucose level, dyslipidaemia, cigarette smoking, and alcohol consumption to Model 2 and conducted multivariable Cox regression analyses (forced entry model). The relationship between systolic blood pressure per 10 mmHg or diastolic blood pressure per 5 mmHg (as a continuous variable) and incident aortic dissection or aortic aneurysm was also analyzed.
Ethics
This study was approved by the Ethical Committee of the University of Tokyo (number: 2018–10862) and was conducted in accordance with the tenets of Declaration of Helsinki. The requirement for informed consent of the present study was waived due to the anonymous nature of the data.
Measurements and definitions
We extracted the following data collected using standardized protocols at the health checkup: BMI, BP, and FPG level. Trained healthcare staff measured the BP at least twice after the participant had been in a resting condition in the Japanese health checkup system, and the average BP was recorded, according to the recommendations of the Ministry of Health, Labour and Welfare and the Japanese Society of Cardiovascular Disease Prevention. Detailed BP measuring methods are summarized in Supplementary material. Consistent with the 2017 ACC/AHA BP guideline,15 we categorized the participants as having normal BP (systolic BP [SBP] < 120 mmHg and diastolic BP [DBP] < 80 mmHg), elevated BP (SBP of 120–129 mmHg and DBP < 80 mmHg), stage 1 hypertension (SBP of 130–139 mmHg or DBP of 80–89 mmHg), and stage 2 hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg). We defined prediabetes as FPG level of 100–125 mg/dL and diabetes as FPG level of ≥126 mg/dL. Dyslipidaemia was defined as low-density lipoprotein cholesterol level of ≥140 mg/dL, high-density lipoprotein cholesterol level of <40 mg/dL, or triglyceride level of ≥150 mg/dL or use of lipid-lowering medications.16 Information on cigarette smoking (current or non-current) and alcohol consumption (daily or not) was obtained from self-reported questionnaires at health checkup.
Outcomes
We collected the outcome data between January 2005 and April 2021. The primary outcomes were AD (ICD-10 code: I710) and AA (ICD-10 codes: I711–1716, I718, and I719).
Statistical analysis
Continuous variables were presented as median (Q1–Q3), and categorical variables were presented as numbers (percentages). We assessed the statistical significance of differences between groups using analysis of variance for continuous variables and χ2 test for categorical variables. The study participants were categorized into nine groups based on the combination of BP classification (normal/elevated BP, Stage 1 hypertension, and Stage 2 hypertension) and glycaemic status (normal FPG, prediabetes, and diabetes). To identify the association of BP category with incident AD or AA, we conducted Cox regression analyses. Model 1 included BP category alone (unadjusted model). Model 2 included BP category, age, and sex, and multivariable Cox regression analyses (forced entry model) were conducted. Furthermore, as in Model 3, we added BMI, FPG level, dyslipidaemia, cigarette smoking, and alcohol consumption to Model 2 and conducted multivariable Cox regression analyses (forced entry model). The relationship between SBP per 10 mmHg or DBP per 5 mmHg (as a continuous variable) and incident AD or AA was also analyzed. Similar to BP category, to identify the association between glycaemic status and the incidence of AD or AA, we conducted Cox regression analyses. Model 1 included the glycaemic status alone (unadjusted model). Model 2 included glycaemic status, age, and sex, and multivariable Cox regression analyses (forced entry model) were conducted. Furthermore, as in Model 3, we added BMI, SBP, dyslipidaemia, cigarette smoking, and alcohol consumption to Model 2 and conducted multivariable Cox regression analyses (forced entry model). The relationship between FPG level per 10 mg/dL (as a continuous variable) and incident AD or AA was also analyzed. To identify the combined effect of BP category and glycaemic status on incident AD or AA, we conducted multivariable Cox regression analyses, including nine categories based on BP category and glycaemic status, age, sex, BMI, dyslipidaemia, cigarette smoking, and alcohol consumption (forced entry model). We assessed the association between continuous changes in SBP and FPG and the risk of developing AD or AA using a restricted cubic spline regression model. We used four cutoff points for SBP and FPG level (5, 35, 65, and 95 percentiles), with the reference point set at 120 mmHg for SBP and 100 mg/dL for FPG level. We fitted three cubic spline models using three, four, and five knots, and the model with four knots was selected because it had the lowest Akaike’s information criterion. Hazard ratios (HRs) and 95% confidence intervals (CIs) for incident AD or AA were calculated and adjusted for the same covariates used in the multivariable Cox regression analyses. We estimated variance inflation factor (VIF) to assess the multicollinearity among the variables of the multivariable Cox proportional hazard models. A generalized additive model was used to visualize the interaction between SBP and FPG level on the risk of development of AD or AA using smoothing splines with SBP and FPG level as nonlinear continuous variables and Cox proportional hazard models. We conducted twelve sensitivity analyses to confirm the combined effect of BP category and glycaemic status on incident AD or AA. First, we performed multiple imputations for the missing data (cigarette smoking and alcohol consumption). Second, because death could be a competing risk of AD or AA events, we performed Fine and Gray's proportional subhazards model as a competing risks analysis. Third, we performed another sensitivity analysis in which we included individuals taking BP- or glucose-lowering medications at the initial health checkup to confirm the association of BP category and FPG category with incident AD or AA. In this sensitivity analysis, we categorized individuals using BP-lowering medications to the Stage 2 hypertension group and those using glucose-lowering medications to the diabetes group. Fourth, we examined the association of BP and FPG with the incidence of AD or AA after additional adjustment of year at the initial health checkup. Fifth, we redefined prediabetes as FPG level of 110–125 mg/dL and diabetes as FPG level of ≥126 mg/dL. Sixth, we redefined the incident of AD or AA requiring aneurysmectomy or stent-graft insertion as outcomes. We excluded individuals having AD or AA diagnoses without aneurysmectomy or stent-graft insertion. Seventh, we examined the associations of BP and FPG with thoracic AA (ICD-10 codes: I711 and I712) or abdominal AA (ICD-10 codes: I713 and I714). Eighth, we extracted information on whether BP- or glucose-lowering medications were prescribed at one year after the initial health checkup. We re-examined the associations of BP and FPG with AD and AA stratified by whether BP- or glucose-lowering medications were prescribed after the initial health checkup. Ninth, although we excluded individuals with history of CVD (myocardial infarction, angina pain, and stroke), renal disease, or dialysis in the primary analysis, we re-examined the association of BP category and FPG category with incident AD or AA without excluding these individuals. Tenth, we examined the association of BP category with incident of AD and AA according to FPG category. Eleventh, we examined the association of FPG category with incident of AD and AA according to BP category. Twelfth, the net reclassification improvement (NRI) was assessed to examine the predictive value of the interaction term between the BP category and FPG category when added to the multivariable model. Statistical significance was set at P-value <0.05. Statistical analyses were conducted using Stata version 17 (StataCorp LLC, College Station, TX, USA).
Results
Baseline characteristics
Baseline clinical characteristics of the study participants are shown in Table 1. The median age of the patients was 43 (36–51) years, and 1 919 546 individuals (57.2%) were men. The median SBP and DBP were 117 (106–127) mmHg and 72 (64–80) mmHg, respectively. Stage 1 hypertension and Stage 2 hypertension were observed in 20.3 and 10.8% of participants, respectively. The median FPG level was 91 (85–97) mg/dL. Prediabetes and diabetes were observed in 16.9 and 1.6% of participants, respectively. The comparison of baseline clinical characteristics between the nine groups according to the combination of BP category and glycaemic status is summarized in Supplementary material online, Table S1.
| . | n = 3 358 293 . |
|---|---|
| Age, years | 43 (36–51) |
| Men, n (%) | 1 919 546 (57.2) |
| Body mass index, kg/m2 | 22.2 (20.1–24.6) |
| Systolic blood pressure, mmHg | 117 (106–127) |
| Diastolic blood pressure, mmHg | 72 (64–80) |
| Blood pressure classification | |
| Normal blood pressure, n (%) | 1 817 256 (54.1) |
| Elevated blood pressure, n (%) | 498 564 (14.8) |
| Stage 1 hypertension, n (%) | 681 199 (20.3) |
| Stage 2 hypertension, n (%) | 361 274 (10.8) |
| Fasting plasma glucose, mg/dL | 91 (85–97) |
| Glycaemic status | |
| Normal fasting plasma glucose, n (%) | 2 736 285 (81.5) |
| Prediabetes, n (%) | 568 501 (16.9) |
| Diabetes, n (%) | 53 507 (1.6) |
| Low-density lipoprotein cholesterol, mg/dL | 118 (98–140) |
| High-density lipoprotein cholesterol, mg/dL | 62 (52–74) |
| Triglycerides, mg/dL | 78 (55–118) |
| Dyslipidaemia, n (%) | 1 239 916 (36.9) |
| Cigarette smoking, n (%) | 855 534 (25.5) |
| Alcohol consumption, n (%) | 721 189 (21.5) |
| . | n = 3 358 293 . |
|---|---|
| Age, years | 43 (36–51) |
| Men, n (%) | 1 919 546 (57.2) |
| Body mass index, kg/m2 | 22.2 (20.1–24.6) |
| Systolic blood pressure, mmHg | 117 (106–127) |
| Diastolic blood pressure, mmHg | 72 (64–80) |
| Blood pressure classification | |
| Normal blood pressure, n (%) | 1 817 256 (54.1) |
| Elevated blood pressure, n (%) | 498 564 (14.8) |
| Stage 1 hypertension, n (%) | 681 199 (20.3) |
| Stage 2 hypertension, n (%) | 361 274 (10.8) |
| Fasting plasma glucose, mg/dL | 91 (85–97) |
| Glycaemic status | |
| Normal fasting plasma glucose, n (%) | 2 736 285 (81.5) |
| Prediabetes, n (%) | 568 501 (16.9) |
| Diabetes, n (%) | 53 507 (1.6) |
| Low-density lipoprotein cholesterol, mg/dL | 118 (98–140) |
| High-density lipoprotein cholesterol, mg/dL | 62 (52–74) |
| Triglycerides, mg/dL | 78 (55–118) |
| Dyslipidaemia, n (%) | 1 239 916 (36.9) |
| Cigarette smoking, n (%) | 855 534 (25.5) |
| Alcohol consumption, n (%) | 721 189 (21.5) |
Data are expressed as number (percentage) or median (Q1–Q3).
| . | n = 3 358 293 . |
|---|---|
| Age, years | 43 (36–51) |
| Men, n (%) | 1 919 546 (57.2) |
| Body mass index, kg/m2 | 22.2 (20.1–24.6) |
| Systolic blood pressure, mmHg | 117 (106–127) |
| Diastolic blood pressure, mmHg | 72 (64–80) |
| Blood pressure classification | |
| Normal blood pressure, n (%) | 1 817 256 (54.1) |
| Elevated blood pressure, n (%) | 498 564 (14.8) |
| Stage 1 hypertension, n (%) | 681 199 (20.3) |
| Stage 2 hypertension, n (%) | 361 274 (10.8) |
| Fasting plasma glucose, mg/dL | 91 (85–97) |
| Glycaemic status | |
| Normal fasting plasma glucose, n (%) | 2 736 285 (81.5) |
| Prediabetes, n (%) | 568 501 (16.9) |
| Diabetes, n (%) | 53 507 (1.6) |
| Low-density lipoprotein cholesterol, mg/dL | 118 (98–140) |
| High-density lipoprotein cholesterol, mg/dL | 62 (52–74) |
| Triglycerides, mg/dL | 78 (55–118) |
| Dyslipidaemia, n (%) | 1 239 916 (36.9) |
| Cigarette smoking, n (%) | 855 534 (25.5) |
| Alcohol consumption, n (%) | 721 189 (21.5) |
| . | n = 3 358 293 . |
|---|---|
| Age, years | 43 (36–51) |
| Men, n (%) | 1 919 546 (57.2) |
| Body mass index, kg/m2 | 22.2 (20.1–24.6) |
| Systolic blood pressure, mmHg | 117 (106–127) |
| Diastolic blood pressure, mmHg | 72 (64–80) |
| Blood pressure classification | |
| Normal blood pressure, n (%) | 1 817 256 (54.1) |
| Elevated blood pressure, n (%) | 498 564 (14.8) |
| Stage 1 hypertension, n (%) | 681 199 (20.3) |
| Stage 2 hypertension, n (%) | 361 274 (10.8) |
| Fasting plasma glucose, mg/dL | 91 (85–97) |
| Glycaemic status | |
| Normal fasting plasma glucose, n (%) | 2 736 285 (81.5) |
| Prediabetes, n (%) | 568 501 (16.9) |
| Diabetes, n (%) | 53 507 (1.6) |
| Low-density lipoprotein cholesterol, mg/dL | 118 (98–140) |
| High-density lipoprotein cholesterol, mg/dL | 62 (52–74) |
| Triglycerides, mg/dL | 78 (55–118) |
| Dyslipidaemia, n (%) | 1 239 916 (36.9) |
| Cigarette smoking, n (%) | 855 534 (25.5) |
| Alcohol consumption, n (%) | 721 189 (21.5) |
Data are expressed as number (percentage) or median (Q1–Q3).
Association of BP category and incident AD and AA
In the mean follow-up of 1 199 ± 950 days, 1 095 and 2 177 cases of AD and AA, respectively, were recorded. Age distribution of cases who developed AD or AA are shown in Supplementary material online, Figure S2. The incidences of AD and AA increased with the BP category. In the multivariable Cox regression analyses (Model 3), compared with normal/elevated BP, HRs of Stage 1 and Stage 2 hypertension were 1.89 (95% CI: 1.60–2.22) and 5.87 (95% CI: 5.03–6.84) for AD and 1.37 (95% CI: 1.23–1.52) and 2.17 (95% CI: 1.95–2.42) for AA, respectively. HRs of SBP per 10 mmHg and DBP per 5 mmHg were 1.43 (95% CI: 1.39–1.48) and 1.40 (95% CI: 1.36–1.43) for AD and 1.17 (95% CI: 1.14–1.20) and 1.15 (95% CI: 1.12–1.17) for AA, respectively (Figure 1). All variables entered in Model 3 had VIF values of <2.5.
Association of glycaemic status and incident AD and AA
The incidence of AD and AA increased with glycaemic status. Similarly, the unadjusted model (Model 1) showed that the risk of developing AD or AA increased with increasing FPG level. However, after adjusting for covariates (Model 3), the risk of AD or AA decreased with glycaemic status. Compared with those of normal FPG level, HRs of prediabetes and diabetes were 0.82 (95% CI: 0.71–0.94) and 0.48 (95% CI: 0.33–0.71) for AD and 0.94 (95% CI: 0.85–1.03) and 0.61 (95% CI: 0.47–0.79) for AA, respectively. HRs of FPG level per 10 mg/dL were 0.89 (95% CI 0.85–0.94) for AD and 0.95 (95% CI 0.92–0.98) for AA, respectively (Figure 2). All variables entered in model 3 had VIF values of <2.5.

Glycaemic status and risk of aortic dissection or aneurysm. The association of fasting plasma glucose category with the risk of aortic dissection (A) and aortic aneurysm (B) is shown. We conducted Cox regression analyses. Model 1 included the glycaemic status alone (unadjusted model). Model 2 included glycaemic status, age, and sex, and multivariable Cox regression analyses (forced entry model) were conducted. Furthermore, as in Model 3, we added body mass index, systolic blood pressure, dyslipidaemia, cigarette smoking, and alcohol consumption to Model 2 and conducted multivariable Cox regression analyses (forced entry model). The relationship between fasting plasma glucose level per 10 mg/dL (as a continuous variable) and incident aortic dissection or aortic aneurysm was also analyzed.
Association of the combination of BP category and glycaemic status with incident AD and AA
We detected significant interactions between the BP category and FPG category for AD (P = 0.0171) and AA (P = 0.0093). Furthermore, compared with normal/elevated BP and normal FPG level, HRs of Stage 2 hypertension and normal FPG level for AD and AA were 6.70 (95% CI: 5.62–7.98) and 2.42 (95% CI: 2.12–2.75), respectively, whereas those of Stage 2 hypertension and diabetes for AD and AA were 2.90 (95% CI: 1.75–4.79) and 1.10 (95% CI: 0.74–1.64), respectively (Figure 3).
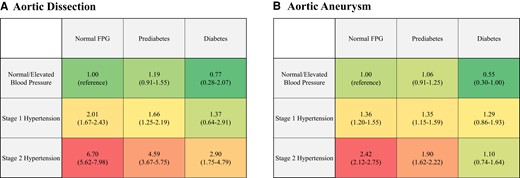
Association with combination of blood pressure and glycaemic status with risk of aortic dissection or aneurysm. Hazard ratios for aortic dissection (A) and aortic aneurysm (B) are summarized. Hazard ratios of each combination based on blood pressure category and fasting plasma glucose category are adjusted for age, sex, body mass index, dyslipidaemia, cigarette smoking, and alcohol consumption. We conducted multivariable Cox regression analyses, including nine categories based on blood pressure category and glycaemic status, age, sex, body mass index, dyslipidaemia, cigarette smoking, and alcohol consumption (forced entry model).
Restricted cubic spline
The risk of AD and AA increased monotonically with SBP after SBP exceeded 110–120 mmHg. The risk of AD and AA decreased with FPG level after FPG exceeded 100 mg/dL (Figure 4).
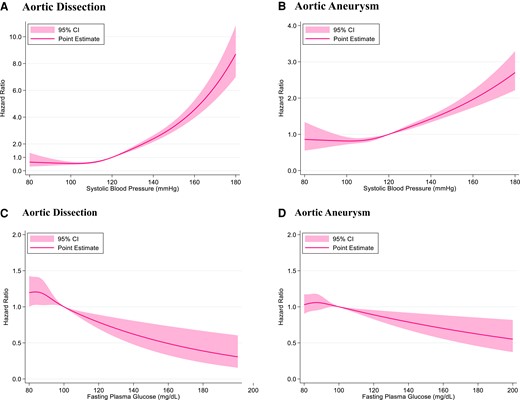
Restricted cubic spline. Restricted cubic spline of systolic blood pressure for aortic dissection (A) and aortic aneurysm (B) is shown. Hazard ratios of systolic blood pressure are adjusted for age, sex, body mass index, fasting plasma glucose level, dyslipidaemia, cigarette smoking, and alcohol consumption. Restricted cubic spline of fasting plasma glucose level for aortic dissection (C) and aortic aneurysm (D) is shown. Hazard ratios of fasting plasma glucose level are adjusted for age, sex, body mass index, systolic blood pressure, dyslipidaemia, cigarette smoking, and alcohol consumption.
Generalized additive model
Contour plots for the interaction between SBP and FPG level showed that higher SBP and lower FPG level were associated with an elevated risk of AD and AA. A lower risk is represented by blue and a higher risk is represented by yellow (Figure 5).
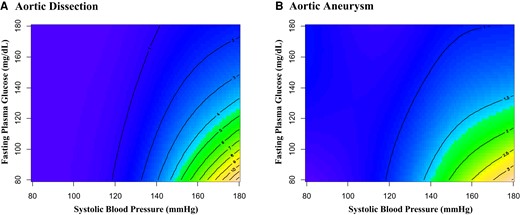
Interaction between systolic blood pressure and fasting plasma glucose on risk for aortic dissection and aortic aneurysm. Contour plots using generalized additive models for interaction between systolic blood pressure and fasting plasma glucose level in the development of aortic dissection (A) and aortic aneurysm (B). Models included age, sex, body mass index, dyslipidaemia, cigarette smoking, alcohol consumption, systolic blood pressure, fasting plasma glucose, and the interaction term between systolic blood pressure and fasting plasma glucose.
Sensitivity analysis
First, we analyzed 3 952 417 individuals after multiple imputations and found that the risk of AD or AA increased with the BP category and decreased with FPG status in this population (see Supplementary material online, Figure S3). Second, the combined effects of BP category and glycaemic status on incident AD or AA were unchanged in the competing risks model (see Supplementary material online, Figure S4). Third, after including individuals using BP- or glucose-lowering medications, we analyzed 3 734 753 individuals. Also in this analysis, the combined effects of BP category and glycaemic status on the risk of developing AD or AA were observed (see Supplementary material online, Figure S5). Fourth, our results were unchanged even after additional adjustment for year at the initial health checkup (see Supplementary material online, Figure S6). Fifth, the combined effects of BP category and glycaemic status on incident AD or AA were unchanged after changing the definition of prediabetes (i.e. FPG 110–125 mg/dL) (see Supplementary material online, Figure S7). Sixth, we defined the incidence of AD or AA requiring aneurysmectomy or stent-graft insertion as outcomes and excluded 2 402 individuals having AD or AA diagnoses without aneurysmectomy or stent-graft insertion. We analyzed 3 thinsp;355 891 individuals, and 395 AD and 422 AA were identified. HRs at each level showed similar trends (see Supplementary material online, Figure S8). Seventh, we examined the associations of BP and FPG with thoracic AA or abdominal AA. Compared with normal/elevated BP and normal FPG level, Stage 2 hypertension and normal FPG level or prediabetes level had a higher risk for thoracic AA and abdominal AA (see Supplementary material online, Figure S9). Eighth, we extracted 105 697 individuals with BP- or glucose-lowering medications at one year after the initial health checkup. We performed subgroup analysis stratified by whether BP- or glucose-lowering medications were prescribed after the initial health checkup. In both subgroups, diabetes levels tended to have lower HRs for the incidence of AD and AA in each BP category (see Supplementary material online, Figures S10 and S11). P for interaction between nine groups and the presence of BP- or glucose-lowering medications for the incidence of AD and AA were 0.1277 and <0.001, respectively. Ninth, we included individuals with history of CVD, renal disease, or dialysis, and analyzed 3 407 751 individuals. The combined effects of BP category and glycaemic status on the risk of developing AD or AA were observed in this case scenario (see Supplementary material online, Figure S12). Tenth, Stage 1 or Stage 2 hypertension had a higher risk for AD and AA compared to the normal/elevated BP at each FPG category (see Supplementary material online, Table S2). Eleventh, compared to normal FPG, diabetes tended to have a lower HR at each BP category (see Supplementary material online, Table S2). Twelfth, the NRI showed that the addition of the interaction term between BP category and FPG category in the multivariable model improved the predictive abilities for the risk of developing AA or AD (see Supplementary material online, Table S3).
Discussion
In this nationwide study, we observed that a dose-dependent increase and decrease in the risk of AD or AA was associated with BP and FPG level, respectively. Although presence of Stage 2 hypertension increased the risk of developing both AD and AA, the risk of incident AD and AA decreased with FPG levels in patients with Stage 2 hypertension. The robustness of our results was confirmed using sensitivity analyses.
Our results showing the positive relationship of hypertension and incident AD or AA and the inverse association of hyperglycaemia and risk of developing AD or AA are generally consistent with those of previous studies.7–14 Various pathological mechanisms can be suggested for the relationship between hypertension and elevated risk of AD or AA. Hypertension can contribute to the development of AD or AA through atherosclerosis.17 Hypertension also increases aortic wall stress, leading to the development of AD and AA. Furthermore, high BP could provoke a loss of elastic fibres in the aortic media and destabilize the elastic lamina connection.18 Once the aorta expands, high BP-associated increasing wall stress would accelerate aortic enlargement in accordance with Laplace’s law. Although the inverse association between hyperglycaemia and the risk of developing AD or AA is paradoxical, there are several possible explanations for this inverse association. The pathophysiological mechanisms associated with diabetes, such as decreased secretion of metalloproteinases; increased plasminogen activator inhibitor-1 generation; decreased adventitial neovascularization; and enhancement of transforming growth factor-β signalling in the aortic wall, would protectively influence the pathogenesis of AD or AA.19–22 For example, hyperglycaemia increases plasminogen activator inhibitor-1 expression and is associated with decreased adventitial neovascularization.22 Conseqently, vascular smooth muscle cell death and extracellular matrix degradation are reduced, which may reduce the development of AD and AA.
The present study is distinguishable from previous studies in the following points: First, although uncontrolled high BP is thought to increase the risk of AD or AA, we found that the risk of developing AD and AA started to increase within the normal range of BP (SBP <130 mmHg), which is in line with a recent meta-analysis.7 Given that the mean follow-up period was relatively short (3.3 years on average), we need to recognize that AD or AA may occur in individuals with lower BP and earlier than expected. Second, compared with people with normal FPG levels, the risk of AD or AA was lower in those with diabetes. The restricted cubic spline showed that the risk of AD and AA continued to decrease with increasing FPG levels without attenuation. In particular, compared with normal FPG levels, not only diabetes but also prediabetes was significantly associated with a lower risk for the incidence of AD. It would be interesting to determine whether treatment for hyperglycaemia (glucose-lowering medication) could influence the subsequent risk of AD or AA. Finally, despite the importance of risk assessment of AD and AA in individuals with hypertension and diabetes, whose coexistence is common, little is known about the interaction between BP and FPG in the development of AD or AA. Therefore, our findings revisit a risk stratification of AA and AD among patients with hypertension and diabetes.
There are several limitations in the present study, most of which are due to the use of the health checkup and administrative database as we described in our earlier publications. BP and FPG level could vary, and a single measurement (e.g. health checkup) cannot reflect the BP or FPG phenotype of study individuals. As discussed above, most AA cases are asymptomatic. Therefore, the incidence of AA might be underestimated, and their attributable risk of death could not be adequately estimated. The JMDC Claims Database primarily included the employed working-age population; therefore, we need to consider selection bias, and it remains unclear whether the results of the present study can be applied to other populations (e.g. different races, older age). The results of this study may need to be re-examined using other independent databases. Although we conducted multivariate Cox regression analyses, the possibility of unmeasured confounding factors could not be eliminated (e.g. socioeconomic status, salt-intake). Because the follow-up period was relatively short, further studies with a long-term follow-up period are needed to confirm our findings. Although experienced medical staff measured BP based on the protocol according to the recommendations of the Japanese Ministry of Health, Labour and Welfare at health checkups, adherence to this recommended protocol in a real-world screening situation on a nationwide scale may be limited. The status of BP/FPG and mediations for hypertension/diabetes could change during the observational period, which could influence the clinical course of study participants. Our results showed the association of hyperglycaemia with a decreased risk of AD or AA. Although we excluded participants taking glucose-lowering medications at baseline from our main analysis, some participants could have started glucose-lowering medications during the observational period, and these medications could have influenced the results.23 Therefore, we need to take this point into consideration when we interpret the results of the present study. The JMDC Claims Database does not have complete information on genetic predisposition. The diagnoses in insurance claims datasets (like ours) should typically be deemed as less thoroughly validated, even though the accuracy of recorded diagnoses (including CVD) in a Japanese administrative claims data was known to be high.22,23 Because we defined outcomes based on the ICD-10 code, we could not extract complete information about the type of AD (Stanford A or Stanford B) and intramural haematoma.
In conclusion, our analysis, which included more than 3 000 000 adults registered in a nationwide health checkup and administrative claims database, demonstrated that the risk of developing AD or AA increased with increasing BP but decreased with increasing FPG level. Even in individuals with Stage 2 hypertension, the subsequent risk of AD or AA decreases with glycaemic status. This is the first study to identify the dose-dependent association among hypertension, hyperglycaemia and the risk of developing AD or AA using a large-scale real-world dataset.
Author contributions
H.K., Y.Y., A.O., H.Y., and I.K. contributed to the conception and design of this study. Y.S., A.O., H.I., S.M., N.M., T.J., and H.Y. analyzed all data. H.K., Y.Y., A.O., K.F., H.M., K.N., H.Y., and I.K. contributed to the interpretation of data. H.K., Y.Y., A.O., T.N., and H.M. drafted the manuscript. T.N., H.M., H.Y., and I.K. contributed to the critical revision for this paper. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology online.
Funding
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907, 21H03159, and 21K08123). The funding sources had nothing regarding the current study.
Data availability
The JMDC Claims Database is available for purchase from JMDC Inc. (https://www.jmdc.co.jp/en/).
References
Author notes
Conflict of interest: Research funding and scholarship funds (Hidehiro Kaneko and Katsuhito Fujiu) from Medtronic Japan; Biotronik Japan; SIMPLEX QUANTUM; Boston Scientific Japan; and Fukuda Denshi, Central Tokyo. Akira Okada is a member of the Department of Prevention of Diabetes and Lifestyle-related Diseases, which is a co-operative programme between The University of Tokyo and Asahi Mutual Life Insurance Company. The remaining authors have nothing to disclose. The funding sources had nothing with regard to the current study.




Comments