-
PDF
- Split View
-
Views
-
Cite
Cite
Maxime Boidin, Gregory Y H Lip, Dick Thijssen, Role of cardiorespiratory fitness in cancer development: time for an update, European Journal of Preventive Cardiology, Volume 28, Issue 17, December 2021, Pages e14–e16, https://doi.org/10.1177/2047487320935228
Close - Share Icon Share
The relationship between cardiorespiratory fitness (CRF) and cardiovascular disease and mortality has been well established.1 However, this relationship has been less well studied with cancer, despite cancer representing the second leading cause of death worldwide.2
Almost half of the cancer incidence is attributable to modifiable risk factors.3 Among these, physical activity, dietary intake and smoking are the main modifiable risk factors for cancer through lifestyle changes.3 Importantly, several studies have found an association between CRF and death-related cancer,4 but these data are limited to men and particular types of cancer, and do not take into account the rapid improvement of cancer treatments. This highlights the need to update the rationale and relevance of targeting CRF in contemporary medicine related to cancer.
In the present issue of the journal, Fardman et al.5 evaluate the impact of midlife CRF on survival following cancer diagnosis after a median follow-up of 13 years in 19,134 healthy men and women with survival data available from the Israeli Population Registry. CRF was indirectly but objectively measured by the Bruce protocol, which is strongly correlated with the direct measure maximal oxygen uptake (peak O2). The 5-minute heart rate recovery was also measured. Thereby, CRF was computed according age and gender-specific distribution, and divided into quintiles of fitness (lower fitness: quantiles 1 and 2; higher fitness: quantiles 3–5). The 10-year Atherosclerotic Cardiovascular Disease Pooled Cohort Equation (ASCVD PCE) risk score was computed for estimation of future cardiovascular disease risk in primary care settings.
From the original cohort, 1455 (7.6%) of the total individuals (50 ± 8 years) developed cancer during the follow-up and 308 (21.2%) of these died. Mean time to cancer diagnosis was 6.6 ± 4.0 years. Low-fitness individuals were 13% more likely to develop cancer during follow-up, and the cumulative probability of death at 6 years from the time of cancer diagnosis was lower among high-fitness patients (19 ± 1% vs 23 ± 2%). The presence of cancer, lower fitness, higher ASCVD score and presence of chronic kidney disease were independent predictors of mortality. Also, individuals with an intermediate baseline CRF (>4 metabolic equivalents (METs) had a lower risk of death after diagnosis of cancer, and each 1 MET increase in peak O2 was associated with a 7% lower risk of mortality from cancer.
What are the implications of this study? The two main finding in this study are that higher CRF is associated with a lower risk of cancer development and cancer-related mortality, and that each increase of the CRF decreases the risk of mortality. This highlights the benefits of regular performance of exercise training on the decrease risk of cancer development and cancer-related mortality, and on the additional increase of CRF which leads to significant clinical benefits. Considering these clinical benefits, it reinforces the need of measuring CRF in a clinical routine. A direct measure of CRF using maximal cardiopulmonary exercise testing is considered as the gold standard, but requires valuable time, equipment and team support. Fardman et al. used the Bruce protocol, an indirect but well-validated test for estimating CRF with maximal exertion, determined as achieving ≥85% age-predicted maximal heart,6 corresponding to approximatively 75% of peak O2.7 Although using these criteria may underestimate the maximal CRF in this study, and perhaps even underestimate the true predictive value, this study showed that even indirect measures of CRF have clinical relevance given its strong, independent prognostic value. At least, these findings highlight that (indirect) measures of CRF have important clinical relevance, and can predict cancer development and mortality.
CRF was determined at baseline, and outcomes ascertained >6 years later. Risks for cancer and other conditions would have been influenced by ageing and incident comorbidities, as well as changes in drug therapies and lifestyle (e.g. smoking, alcohol consumption and even CRF) over the time period. Whilst the association between a baseline variable and adverse outcome(s) many years later is interesting, the reality is that risk is not a static ‘one off’ measure, but a dynamic process requiring risk re-assessment(s) during follow-up. The ability to assess CRF with relatively simple, non-invasive (in)direct measures adds to the strength and clinical value of repeatedly measuring CRF in clinical practice.
CRF and cancer or cancer-related mortality
According the Fick equation, CRF (or directly peak O2) is determined by the product of cardiac output and arterio-venous oxygen difference, where cardiac output is the product of stroke volume and heart rate. Consequently, when one or several of these determinants increase, CRF increases, and exercise training is often used to increase these determinants. However, a higher CRF is not only just explained by exercise training, but (arguably) ∼50% by genetic factor(s).8 Moreover, there may be an association between physical activity and genes, where physical activity may have an impact on the genetic component, and vice versa.9 In the end, the protective effect of higher CRF on cancer development and cancer-related mortality could be determined by the combination of a modifiable behaviour (i.e. exercise training/physical activity) and a non-modifiable component (i.e. genetic background), but also the interaction between both (Figure 1).
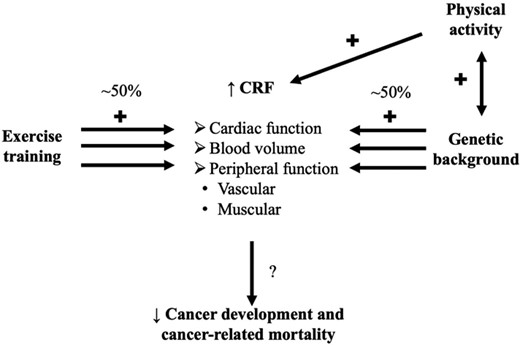
Representation of the relation between cardiorespiratory fitness (CRF) and its determinants, and cancer/cancer-related mortality.
Perspectives
Treatments for cancer may involve surgery, radiation, chemotherapy, hormones and immunotherapy. In the process of destroying cancer cells, some treatments also damage healthy tissue. Individuals with cancer may experience side-effects that limit their ability to exercise during treatment and afterwards.10 Therefore, peak O2 and cardiac function are reduced and troponin, an index of cardiotoxicity, is increased during chemotherapy.11 Furthermore, overall physical function is generally diminished because of losses of aerobic capacity, muscle tissue, and range of motion. Even among cancer survivors who are 5 years or more post-treatment, more than 50% report physical capacity limitations including kneeling, lifting, standing and walking.12 Consequently, baseline CRF may be lower in non-survivors compared with survivors and, secondly, CRF is strongly altered after cancer diagnosis because of the cancer itself and the related cancer therapy.10 However, the decrease in peak O2 during chemotherapy could be attenuated, but not improved, with continuous exercise training.13 Potential benefits of exercise training in individuals with cancer-related cardiotoxicity include reduction of secondary cancer, cancer relapse risk, cardiovascular complications and disease. Exercise training prescription should be personalised according to the profile, the interest and the response to training of the individual, and adapted according the treatment over time.14
In conclusion, the authors should be congratulated for addressing the strong association between baseline CRF and survival. This indicates that modifiable risk factors such as physical activity could be a target to prevent cancer development, and to prevent the consequences of cancer and cancer-related treatment. This study adds further evidence not only for the rationale of measuring and targeting CRF as a strategy in the primary prevention of cancer, but also to prevent CRF decline after cancer diagnosis.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship and/or publication of this article.
References
World Health Organization.
Fardman A, Banschick GD, Rabia R, et al. Cardiorespiratory fitness and survival following cancer diagnosis. Eur J Prev Cardiol 2020. Epub ahead of print, DOI: 10.1177/2047487320930873.




Comments