-
PDF
- Split View
-
Views
-
Cite
Cite
Ioannis D Laoutaris, Exercise intolerance and skeletal muscle metaboreflex activity in chronic heart failure: Do we need to recruit more muscle in exercise training?, European Journal of Preventive Cardiology, Volume 27, Issue 17, 1 November 2020, Pages 1858–1861, https://doi.org/10.1177/2047487320912623
Close - Share Icon Share
The haemodynamic hypothesis was dominant in chronic heart failure for most of the last century. This hypothesis attributes exercise intolerance characterized by exertional dyspnoea and/or muscle fatigue to impairment in ventricular performance. The more recent neurohormonal, muscle and immunohumoral hypotheses describe chronic heart failure not only in terms of haemodynamic adaptations, but also by neurohormonal overactivation associated with a sympathetic overdrive, activation of the immune response, systemic inflammation and a catabolic state with muscle atrophy, which may be enhanced by disuse and immobilization in advanced stages of disease.
Current paper
In the current issue of this journal, Angius and Crisafuli1 review the literature for the role of an exaggerated feedback from group III/IV muscle afferents in the genesis of fatigue by bringing forward the muscle hypothesis. The muscle hypothesis describes changes in skeletal muscle metabolism, structure and function where an increased activation of metaboreceptors (and chemoreceptors responding to changes in blood gas concentrations) sensitive to the metabolic changes occurring during exercise produces an exaggerated feedback from group III/IV muscle afferents (termed the metaboreflex). The metaboreflex has been associated with increased sympathetic activity, abnormal responses to exercise and with the progression of the syndrome.2,3
Although central fatigue needs to be further illuminated in patients with chronic heart failure (CHF), a vasoconstriction-mediated response to peripheral signals originating from type III/IV muscle afferents in the muscle potentially restrains muscle perfusion and probably contributes to the early development of peripheral muscle fatigue and exercise intolerance shown by patients CHF.2,,,–6
Although Angius and Crisafuli1 focus on muscle fatigue, another aspect that needs to be reported is the role of the metaboreflex in the genesis of dyspnoea, which appears to be at least partially responsible for exercise intolerance in patients with CHF. The reflex network arising from the exercising muscle not only activates autonomic circuits, but also medullary ventilatory centres resulting in an excessive ventilatory response to exercise that presents as exertional dyspnoea.3,4
Most interestingly, muscle fatigue, dyspnoea and exercise intolerance appear to be closely associated not only with the limb muscle, but also with respiratory muscle dysfunction,7 expressed by decreases in inspiratory muscle strength (PImax), but also mainly by the inability of the diaphragm to sustain inspiratory pressure over time, known as sustained PImax (SPImax), i.e. inspiratory work capacity.8 Thus, the tension–time index of the diaphragm (TTIdi) at peak exercise was 0.10 in patients with advanced CHF compared with 0.03 in healthy participants, suggesting a potential early respiratory muscle fatigue for patients with CHF.7 Furthermore, reducing the increased work of respiratory muscles with a ventilator during exercise resulted in a decrease in sympathetic activity and an increase in limb blood flow in patients with CHF, suggesting the modification of an increased diaphragmatic metaboreflex activity,9 similar to the limb muscles. In addition, another study showed that non-invasive ventilatory support combined with aerobic and resistance training provided additional benefits for dyspnoea and quality of life in this population.10 This response seems to be mediated by the metabolic stimulation of small afferent fibres types III and IV from the respiratory muscles, especially from the diaphragm (Figure 1).
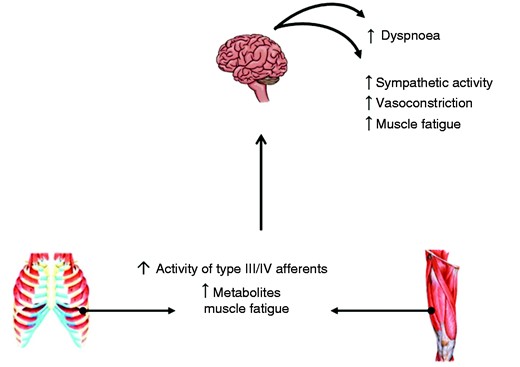
Skeletal muscle (limb and respiratory) metaboreflex activated during fatiguing muscle work due to the accumulation of metabolites and increased activity of type III/IV afferents ,resulting in dyspnoea and increased sympathetic activity and vasoconstriction, exacerbating muscle fatigue and contributing to exercise intolerance.
Angius and Crisafuli1 correctly include recent studies reporting increased activation of the metaboreflex in patients using beta blockers11,12 compared with previous studies (beta blockers in ≤30% of patients).3,4,6 Based on the consideration that the metaboreflex is mediated by the sympathetic nervous system and that beta blockers are adrenergic blockers, they could affect their hyperactivation by decreasing the sympathetic tonus, potentially influencing the metaboreflex activation.
These findings, in conjunction with the evidence of the metaboreflex activity arising from the diaphragm, support the muscle hypothesis and the role of the metaboreflex as a contributor to exercise intolerance in patients with CHF on optimum medical therapy.
Exercise training and skeletal muscle metaboreflex activity
Modification of the exaggerated metaboreflex activity with aerobic training has been suggested as one of the main mechanisms of improvement in exercise tolerance and quality of life in patients with CHF. Aerobic training has been shown to improve the sympathovagal and ventilatory responses to exercise in patients with CHF,13 a finding that appears to be mediated by a reduction in the exaggerated metaboreflex.3 A recent study showed that aerobic training resulted in an improvement in muscle metaboreflex and mechanoreflex control of muscle sympathetic nerve activity, which was associated with an improvement in peak oxygen consumption (peakVO2).14 However, a number of studies are increasingly adding either resistance training or inspiratory muscle training (IMT) to aerobic training (moderate intensity or high-intensity interval), reporting significantly enhanced benefits in exercise tolerance in patients with CHF.
Selective IMT has been shown to result in the amelioration of dyspnoea15,16 and there is increasing evidence for an IMT-induced delay in diaphragmatic metaboreflex activity and an increase in limb blood flow in patients with CHF.17,18 Another study reported decreased sympathetic activity after IMT19 and there is evidence for improvements in peakVO2 when IMT is performed at a higher training intensity as a percentage of inspiratory muscle work capacity (SPImax) or in patients with severe inspiratory muscle weakness.15,–17 The randomized multicentre Vent-HeFT trial demonstrated that combined aerobic training/IMT provided additional benefits in SPImax, dyspnoea and in functional and serum biomarkers in patients with moderate CHF.20 Selective resistance training of moderate intensity resulted in improvements in heart rate variability, limb blood flow, the mitochondrial ATP production rate and peakVO2,21,22 whereas combined aerobic training/resistance training resulted in additional benefits not only in muscle strength and function, but also in flow-mediated vasodilation and ventilatory and metabolic efficiency in patients with CHF.23,24 Whether these changes are associated with a potential attenuation of metaboreflex activity needs to be investigated. The triple combination of aerobic training/resistance training/IMT (ARIS hypothesis) resulted in enhanced benefits in dyspnoea, respiratory and limb muscle function, cardiopulmonary exercise parameters and quality of life in patients with CHF.25,26
Based on these observations, the recruitment of more muscle in exercise training appears to be associated with enhanced benefits in patients with CHF, with the attenuation of metaboreflex hyperactivity arising as an attractive mechanism for improvement. However, Angius and Crisafuli1 rightfully suggest that further studies are needed to evaluate the effect of metaboreflex activity in producing exaggerated ventilatory responses, central and peripheral fatigue in patients with CHF, and also its potential attenuation in response to different and combined exercise training modalities.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
The author(s) received no financial support for the research, authorship, and/or publication of this article.




Comments