-
PDF
- Split View
-
Views
-
Cite
Cite
Harm Wienbergen, Andreas Fach, Sven Meyer, Jochen Meyer, Janina Stehmeier, Tina Backhaus, Stephan Michel, Kirsten Krämer, Rico Osteresch, Johannes Schmucker, Henning Haase, Tobias Härle, Albrecht Elsässer, Rainer Hambrecht, Effects of an intensive long-term prevention programme after myocardial infarction – a randomized trial, European Journal of Preventive Cardiology, Volume 26, Issue 5, 1 March 2019, Pages 522–530, https://doi.org/10.1177/2047487318781109
Close - Share Icon Share
Abstract
Long-term risk factor control after myocardial infarction (MI) is currently inadequate and there is an unmet need for effective secondary prevention programmes.
It was the aim of the study to compare a 12-month intensive prevention programme (IPP), coordinated by prevention assistants and including education sessions, telephone visits and telemetric risk factor control, with usual care after MI. Three hundred and ten patients were randomized to IPP vs. usual care one month after hospital discharge for MI in two German heart centres. Primary study endpoint was the IPP Prevention Score (0–15 points) quantifying global risk factor control.
Global risk factor control was strongly improved directly after MI before the beginning of the randomized study (30% increase IPP Prevention Score). During the 12-month course of the randomized trial the IPP Prevention Score was improved by a further 14.3% in the IPP group (p < 0.001), while it decreased by 11.8% in the usual care group (p < 0.001). IPP significantly reduced smoking, low-density lipoprotein cholesterol, systolic blood pressure and physical inactivity compared with usual care (p < 0.05). Step counters with online documentation were used by the majority of patients (80%). Quality of life was significantly improved by IPP (p < 0.05). The composite endpoint of adverse clinical events was slightly lower in the IPP group during 12 months (13.8% vs. 18.9%, p = 0.25).
A novel intensive prevention programme after MI, coordinated by prevention assistants and using personal teachings and telemetric strategies for 12 months, was significantly superior to usual care in providing sustainable risk factor control and better quality of life.
Introduction
Guideline-recommended prevention is crucial to reduce coronary morbidity and mortality after myocardial infarction (MI);1 however, ‘real-world’ studies, such as the EUROASPIRE surveys, have shown that cardiovascular risk factors are insufficiently controlled in clinical practice and there is an unmet need for implementation of effective long-term prevention programmes after coronary events.2,3 Different studies on prevention programmes have been performed. In the EUROACTION trial (2003–2006) a 16 week nurse-coordinated prevention programme was associated with a trend towards better smoking prevention and lower low-density lipoprotein (LDL) cholesterol; however, there were no relevant effects on total cholesterol or body weight.4 More current studies showed inconsistent results in prevention programmes.5–9
In Germany, most patients with MI are transferred after the acute hospital stay for a three-week period of cardiac rehabilitation; however, follow-up examinations observed disappointing results on sustainable cardiovascular risk factor control one year after such short-term cardiac rehabilitation programmes.10
It was the aim of the present study to evaluate the effects of a novel long-term intensive prevention programme (IPP) that included group education sessions, telephone contacts and telemetric control of risk factors and that was coordinated by a non-physician prevention assistant.
Methods
Study population and study design
At two large German heart centres all patients hospitalized for acute ST-elevation myocardial infarction (STEMI) or non-STEMI-elevation myocardial infarction (NSTEMI) were screened for eligibility. Exclusion criteria were: inability to participate in a prevention programme (i.e. due to language barrier), <18 or >75 years of age and any major non-cardiac condition that would adversely affect survival during the duration of the study. Patients with coronary artery bypass surgery (CABG) in the first 30 days after MI were excluded from the study, because compared with the majority of interventionally treated patients substantial differences in the early rehabilitation process (for instance regarding physical activity, quality of life) could be expected.
One month after discharge, mostly (96%) after a three-week cardiac rehabilitation programme, patients were revisited and assigned to IPP for 12 months or to usual care by 1:1 randomization. Follow-up visits were performed after six and 12 months.
Recruitment started in November 2013 and was finished in March 2016; the last patient completed the 12-month study course in March 2017.
The study complies with the Declaration of Helsinki requirements. It was approved by the local ethical committees (Ärztekammer Bremen, Ärztekammer Niedersachsen, Germany). All patients provided written informed consent. ClinicalTrials.gov Identifier is NCT01896765.
IPP
In addition to usual care, patients in the IPP group were educated and controlled to change their lifestyle in a way to reduce cardiovascular risk factors and maintain or optimize medical secondary prevention therapy:
The prevention assistant organized regular group education sessions for each patient every four weeks (12 modules with lectures and practical training on risk factors, medication and emergency training) (Supplementary Material Table 1 online). Lectures and training were performed by physicians, dietitians, physiotherapists and the prevention assistant. The maximal group number was 25 persons/session.
Every three weeks the prevention assistant had personal telephone contact with the patient. Following a standardized protocol the patient was asked for cardiovascular risk factors (smoking, blood pressure, physical inactivity, etc.), medication and any cardiac problems. A cardiologist supervised the prevention assistant with respect to these telephone calls. A telephone hotline was established for any case of cardiac problems.
If insufficiently controlled risk factors were identified, the prevention assistant organized short-term clinical visits at the study centre. At these visits a physician and the prevention assistant again controlled the risk factors and if guideline-recommended targets were not reached, the patients, their general practitioners and cardiologists were requested both orally and in writing to escalate prevention efforts; for instance, to escalate lipid-lowering therapy, if LDL cholesterol levels did not meet the recommended values.
All patients in the IPP group were asked to assess and control physical activity by telemetric devices with online documentation. Patients could choose step counters (Incutex® step counters) with manual online data input by the patients (since November 2013) and/or activity trackers (Medisana® ViFit) with direct data transmission to a study online portal that were added to the study in February 2015. Patients were asked to transmit their activity data at least every week. They received regular feedback information by the study centre. In addition email communication on patients’ risk factors was provided, if patients gave consent for this.
Usual care
Patients in the usual care group received medical and interventional therapy following the actual standard of care. All study participants could participate in specific disease management programmes that are offered by general practitioners in the German health care system for coronary artery diesease (CAD) patients. In the IPP group 45.7% (n = 63) and in the usual care group 52.4% (n = 75) participated in these programmes (p = 0.26).
Study endpoints
The primary endpoint global cardiovascular risk factor control was assessed by the IPP Prevention Score, evaluating six major risk factors: smoking status, LDL cholesterol, physical activity, blood pressure, body mass index and HbA1c (Supplementary Material Table 2). This new score was used because until now no scores focusing on assessment and control of global guideline-recommended risk factor targets in secondary prevention after MI have been developed. The score was based on current risk factor targets of the European guidelines of cardiovascular prevention.1 Point score was developed on the basis of published data on risk reduction by risk factor control, weighting the strong effects of smoking cessation11,12 and reduction of LDL cholesterol1,13 as well as physical activity programmes.14–17 The maximal score is 15 points if all risk factors reach the guideline-recommended targets, the minimal score is 0 points.
Secondary study endpoints were single risk factors, serious adverse events, medical treatment and quality of life (measured by EQ-5D-5L and PHQ-9).
Laboratory analyses
Laboratory analyses were performed at the two participating heart centres, including standard parameters creatinine, haemoglobin and leukocytes as well as risk factor assessment with lipids (such as total cholesterol, LDL cholesterol), HbA1c and serum cotinine to control smoking status.
Statistical analysis
The study hypothesis was that IPP significantly improves global risk factors, measured by the IPP Prevention Score. Based on data of previous prevention trials, in particular the coronary heart disease cohort of the EUROACTION trial, the pre-estimated change of the IPP Prevention Score in the intervention group versus usual care was 13% (1.2 ± 2.8 points) to detect significant results with 90% power and significance level α = 5%.
Baseline characteristics were given as mean values ± standard deviation for continuous variables and absolute numbers and percentages for categorical variables. Analysis of caloric expenditure was performed by medians and quartiles. Baseline parameters, clinical events and medication during 12 months were compared between the groups using the Pearson Chi-square-test and Fisher’s exact test (for rare events) for categorical variables as well as the t-test and Mann–Whitney U-test for continuous variables. Analysis of risk factors during the study course was performed using the t-test (parametric variables), Wilcoxon test (non-parametric variables) and Friedman analysis of variance test (non-parametric variables, >2 parameters) as well as the Mann–Whitney U-test (inter-group analyses). A binary logistic regression model was performed for adjusted analysis of risk factor targets after 12 months.
In all analyses p-values < 0.05 were considered as statistically significant. Statistics were performed by using SPSS (IBM, version 22, 2014).
Results
Screening failures, randomized groups and drop outs
Out of 420 screened patients 110 patients (26%) were not eligible for randomization (screening failures). Most of these patients (n = 105, 25%) refused participation in the study, a minority of patients died (n = 3, 0.7%) or developed exclusion criteria (coronary artery bypass surgery, n = 2, 0.5%). The patients who refused participation were younger (53.2 ± 8.7 vs. 56.5 ± 9.7 years, p < 0.01) and more often smokers at time of admission (66.7% vs. 46.1%, p < 0.01) compared with study participants. No significant differences were found regarding gender (21.8% vs. 18.5% female, p = 0.47), rate of coronary three-vessel disease (18.2% vs. 22.1%, p = 0.36) or body mass index on admission (28.5 ± 4.8 kg/m2 vs. 28.3 ± 3.9 kg/m2, p = 0.67).
Finally, 310 patients were randomly assigned to the groups IPP (n = 155) and usual care (n = 155). Nearly all patients (95.7% IPP, 95.8% usual care) participated in a three-week cardiac rehabilitation programme between hospitalization for treatment of MI and randomization.
After randomization 16 patients (10.3%) in the IPP group and 10 patients (6.5%) in the usual care group refused further participation in the prevention programme or in the study, three patients died; consecutively 138 patients of the IPP group and 143 patients of the usual care group completed the study after 12 months (Supplementary Figure 1).
Clinical characteristics at time of randomization
At time of randomization clinical characteristics were well balanced between both groups regarding age, gender, rate of STEMIs or ejection fraction (all p > 0.05). A lower rate of beta-blocker and ACE inhibitor use was observed in the IPP group. With respect to cardiovascular risk factors the rate of smokers was slightly lower in the IPP group compared with the usual care group without significance (12.3% vs. 21.0%, p = 0.05); the other risk factors and the IPP Prevention Score were well balanced between the groups (Table 1).
| . | Intensive prevention n = 138 . | Usual care n = 143 . |
|---|---|---|
| Age, years MV ± SD | 56.5 ± 10.3 | 56.5 ± 9.1 |
| Male gender n (%) | 112 (81.2) | 117 (81.8) |
| ST-elevation myocardial infarction n (%) | 94 (68.1) | 103 (72.0) |
| Coronary three-vessel disease n (%) | 33 (23.9) | 29 (20.3) |
| Previous diagnosis of CAD n (%) | 8 (5.8) | 12 (8.4) |
| HFrEF n (%) | 6 (4.3) | 5 (3.5) |
| HFmrEF n (%) | 13 (9.4) | 16 (11.2) |
| Medication | ||
| Prasugrel n (%) | 90 (65.2) | 98 (68.5) |
| Ticagrelor n (%) | 41 (29.7) | 34 (23.8) |
| Clopidogrel n (%) | 7 (5.1) | 11 (7.7) |
| Beta-blockers n (%) | 89 (64.5) | 112 (78.3) |
| ACE inhibitors n (%) | 110 (79.7) | 128 (89.5) |
| Statins n (%) | 134 (97.1) | 137 (95.8) |
| Risk factors | ||
| Active smoking n (%) | 17 (12.3) | 30 (21.0) |
| LDL cholesterol, mg/dl MV ± SD | 73 ± 26 | 74 ± 26 |
| Leisure-time physical activity kcal/week med. (quart.) | 704 (0;1800) | 503 (0;1680) |
| Systolic blood pressure, mmHg MV ± SD | 133 ± 17 | 132 ± 16 |
| Diastolic blood pressure, mmHg MV ± SD | 80 ± 10 | 81 ± 10 |
| Body mass index, kg/m2 MV ± SD | 28.1 ± 3.8 | 27.9 ± 3.8 |
| Diabetes mellitus n (%) | 13 (9.4) | 19 (13.3) |
| HbA1c, % MV ± SD | 5.7 ± 0.7 | 5.5 ± 0.4 |
| IPP Prevention Score, points MV ± SD | 10.3 ± 2.1 | 10.2 ± 2.1 |
| . | Intensive prevention n = 138 . | Usual care n = 143 . |
|---|---|---|
| Age, years MV ± SD | 56.5 ± 10.3 | 56.5 ± 9.1 |
| Male gender n (%) | 112 (81.2) | 117 (81.8) |
| ST-elevation myocardial infarction n (%) | 94 (68.1) | 103 (72.0) |
| Coronary three-vessel disease n (%) | 33 (23.9) | 29 (20.3) |
| Previous diagnosis of CAD n (%) | 8 (5.8) | 12 (8.4) |
| HFrEF n (%) | 6 (4.3) | 5 (3.5) |
| HFmrEF n (%) | 13 (9.4) | 16 (11.2) |
| Medication | ||
| Prasugrel n (%) | 90 (65.2) | 98 (68.5) |
| Ticagrelor n (%) | 41 (29.7) | 34 (23.8) |
| Clopidogrel n (%) | 7 (5.1) | 11 (7.7) |
| Beta-blockers n (%) | 89 (64.5) | 112 (78.3) |
| ACE inhibitors n (%) | 110 (79.7) | 128 (89.5) |
| Statins n (%) | 134 (97.1) | 137 (95.8) |
| Risk factors | ||
| Active smoking n (%) | 17 (12.3) | 30 (21.0) |
| LDL cholesterol, mg/dl MV ± SD | 73 ± 26 | 74 ± 26 |
| Leisure-time physical activity kcal/week med. (quart.) | 704 (0;1800) | 503 (0;1680) |
| Systolic blood pressure, mmHg MV ± SD | 133 ± 17 | 132 ± 16 |
| Diastolic blood pressure, mmHg MV ± SD | 80 ± 10 | 81 ± 10 |
| Body mass index, kg/m2 MV ± SD | 28.1 ± 3.8 | 27.9 ± 3.8 |
| Diabetes mellitus n (%) | 13 (9.4) | 19 (13.3) |
| HbA1c, % MV ± SD | 5.7 ± 0.7 | 5.5 ± 0.4 |
| IPP Prevention Score, points MV ± SD | 10.3 ± 2.1 | 10.2 ± 2.1 |
Data are absolute numbers and percentages, mean values ± standard deviation (MV ± SD), medians (med.) and quartiles (quart.). There were no significant between-group differences except for beta-blocker (p 0.01) and ACE inhibitor use (p 0.02).
CAD: coronary artery disease; HFrEF: heart failure with reduced ejection fraction; HFmrEF: heart failure with mid-range ejection fraction; ACE: angiotensin-converting enzyme; LDL: low-density lipoprotein; IPP: intensive prevention programme.
| . | Intensive prevention n = 138 . | Usual care n = 143 . |
|---|---|---|
| Age, years MV ± SD | 56.5 ± 10.3 | 56.5 ± 9.1 |
| Male gender n (%) | 112 (81.2) | 117 (81.8) |
| ST-elevation myocardial infarction n (%) | 94 (68.1) | 103 (72.0) |
| Coronary three-vessel disease n (%) | 33 (23.9) | 29 (20.3) |
| Previous diagnosis of CAD n (%) | 8 (5.8) | 12 (8.4) |
| HFrEF n (%) | 6 (4.3) | 5 (3.5) |
| HFmrEF n (%) | 13 (9.4) | 16 (11.2) |
| Medication | ||
| Prasugrel n (%) | 90 (65.2) | 98 (68.5) |
| Ticagrelor n (%) | 41 (29.7) | 34 (23.8) |
| Clopidogrel n (%) | 7 (5.1) | 11 (7.7) |
| Beta-blockers n (%) | 89 (64.5) | 112 (78.3) |
| ACE inhibitors n (%) | 110 (79.7) | 128 (89.5) |
| Statins n (%) | 134 (97.1) | 137 (95.8) |
| Risk factors | ||
| Active smoking n (%) | 17 (12.3) | 30 (21.0) |
| LDL cholesterol, mg/dl MV ± SD | 73 ± 26 | 74 ± 26 |
| Leisure-time physical activity kcal/week med. (quart.) | 704 (0;1800) | 503 (0;1680) |
| Systolic blood pressure, mmHg MV ± SD | 133 ± 17 | 132 ± 16 |
| Diastolic blood pressure, mmHg MV ± SD | 80 ± 10 | 81 ± 10 |
| Body mass index, kg/m2 MV ± SD | 28.1 ± 3.8 | 27.9 ± 3.8 |
| Diabetes mellitus n (%) | 13 (9.4) | 19 (13.3) |
| HbA1c, % MV ± SD | 5.7 ± 0.7 | 5.5 ± 0.4 |
| IPP Prevention Score, points MV ± SD | 10.3 ± 2.1 | 10.2 ± 2.1 |
| . | Intensive prevention n = 138 . | Usual care n = 143 . |
|---|---|---|
| Age, years MV ± SD | 56.5 ± 10.3 | 56.5 ± 9.1 |
| Male gender n (%) | 112 (81.2) | 117 (81.8) |
| ST-elevation myocardial infarction n (%) | 94 (68.1) | 103 (72.0) |
| Coronary three-vessel disease n (%) | 33 (23.9) | 29 (20.3) |
| Previous diagnosis of CAD n (%) | 8 (5.8) | 12 (8.4) |
| HFrEF n (%) | 6 (4.3) | 5 (3.5) |
| HFmrEF n (%) | 13 (9.4) | 16 (11.2) |
| Medication | ||
| Prasugrel n (%) | 90 (65.2) | 98 (68.5) |
| Ticagrelor n (%) | 41 (29.7) | 34 (23.8) |
| Clopidogrel n (%) | 7 (5.1) | 11 (7.7) |
| Beta-blockers n (%) | 89 (64.5) | 112 (78.3) |
| ACE inhibitors n (%) | 110 (79.7) | 128 (89.5) |
| Statins n (%) | 134 (97.1) | 137 (95.8) |
| Risk factors | ||
| Active smoking n (%) | 17 (12.3) | 30 (21.0) |
| LDL cholesterol, mg/dl MV ± SD | 73 ± 26 | 74 ± 26 |
| Leisure-time physical activity kcal/week med. (quart.) | 704 (0;1800) | 503 (0;1680) |
| Systolic blood pressure, mmHg MV ± SD | 133 ± 17 | 132 ± 16 |
| Diastolic blood pressure, mmHg MV ± SD | 80 ± 10 | 81 ± 10 |
| Body mass index, kg/m2 MV ± SD | 28.1 ± 3.8 | 27.9 ± 3.8 |
| Diabetes mellitus n (%) | 13 (9.4) | 19 (13.3) |
| HbA1c, % MV ± SD | 5.7 ± 0.7 | 5.5 ± 0.4 |
| IPP Prevention Score, points MV ± SD | 10.3 ± 2.1 | 10.2 ± 2.1 |
Data are absolute numbers and percentages, mean values ± standard deviation (MV ± SD), medians (med.) and quartiles (quart.). There were no significant between-group differences except for beta-blocker (p 0.01) and ACE inhibitor use (p 0.02).
CAD: coronary artery disease; HFrEF: heart failure with reduced ejection fraction; HFmrEF: heart failure with mid-range ejection fraction; ACE: angiotensin-converting enzyme; LDL: low-density lipoprotein; IPP: intensive prevention programme.
Global risk factor control during study course
In the first period after MI before randomization, during acute hospital stay and three-week cardiac rehabilitation, global risk factor control was strongly improved (IPP Prevention Score 30% increased) (Figure 1).
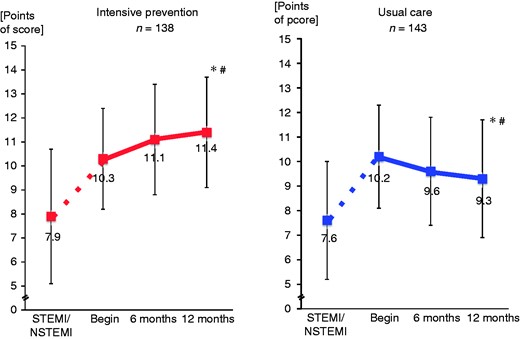
IPP Prevention Score during study course.
*p < 0.001 vs. beginning of study.
#p < 0.001 vs. control cohort.
STEMI: ST-elevation myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction.
Thereafter, from the beginning of the randomized study – one month after discharge – during the next 12 months, the IPP Prevention Score was improved by a further 14.3% in the IPP group, with an increase from 10.3 ± 2.1 points to 11.4 ± 2.3 points (p < 0.001 vs. beginning and vs. usual care). In the usual care group a decrease by 11.8% from 10.2 ± 2.1 points to 9.3 ± 2.4 points was observed (p < 0.001 vs. beginning) (Figure 1).
Single risk factor control
From beginning of study over the next 12 months the rate of recurrent smokers was 3.2% in the IPP group compared with 16.4% in the usual care group (p = 0.017).
Regarding the risk factors LDL cholesterol, total cholesterol and systolic blood pressure significant reductions were achieved by IPP compared with usual care during 12 months (p < 0.05 IPP vs. usual care) (Table 2). Body mass index was not significantly improved in the IPP group; however, there was a significant deterioration in the usual care group (Table 2).
Comparison of risk factors during 12 months, intensive prevention program versus usual care.
| . | Beginning of study . | 12 months . |
|---|---|---|
| LDL cholesterol, mg/dl | ||
| MV ± SD | ||
| – IPP | 73.1 ± 26 | 67.6 ± 21*,# |
| – UC | 73.8 ± 26 | 78.4 ± 29 |
| Total cholesterol, mg/dl | ||
| MV ± SD | ||
| – IPP | 146.7 ± 30 | 143.2 ± 26# |
| – UC | 145.5 ± 28 | 153.3 ± 34* |
| Systolic blood pressure, mmHg | ||
| MV ± SD | ||
| – IPP | 133 ± 17 | 130 ± 15*,# |
| – UC | 132 ± 16 | 135 ± 18* |
| Diastolic blood pressure, mmHg | ||
| MV ± SD | ||
| – IPP | 80 ± 10 | 79 ± 9 |
| – UC | 81 ± 10 | 82 ± 12 |
| Body mass index, kg/m2 | ||
| MV ± SD | ||
| – IPP | 28.1 ± 3.8 | 28.0 ± 4.0 |
| – UC | 27.9 ± 3.7 | 28.3 ± 4.2* |
| HbA1c in diabetics, % | ||
| MV ± SD | ||
| – IPP | 6.7 ± 0.8 | 6.9 ± 0.7 |
| – UC | 6.7 ± 0.8 | 7.3 ± 1.8 |
| . | Beginning of study . | 12 months . |
|---|---|---|
| LDL cholesterol, mg/dl | ||
| MV ± SD | ||
| – IPP | 73.1 ± 26 | 67.6 ± 21*,# |
| – UC | 73.8 ± 26 | 78.4 ± 29 |
| Total cholesterol, mg/dl | ||
| MV ± SD | ||
| – IPP | 146.7 ± 30 | 143.2 ± 26# |
| – UC | 145.5 ± 28 | 153.3 ± 34* |
| Systolic blood pressure, mmHg | ||
| MV ± SD | ||
| – IPP | 133 ± 17 | 130 ± 15*,# |
| – UC | 132 ± 16 | 135 ± 18* |
| Diastolic blood pressure, mmHg | ||
| MV ± SD | ||
| – IPP | 80 ± 10 | 79 ± 9 |
| – UC | 81 ± 10 | 82 ± 12 |
| Body mass index, kg/m2 | ||
| MV ± SD | ||
| – IPP | 28.1 ± 3.8 | 28.0 ± 4.0 |
| – UC | 27.9 ± 3.7 | 28.3 ± 4.2* |
| HbA1c in diabetics, % | ||
| MV ± SD | ||
| – IPP | 6.7 ± 0.8 | 6.9 ± 0.7 |
| – UC | 6.7 ± 0.8 | 7.3 ± 1.8 |
Data are mean values ± standard deviations (MV ± SD).
LDL: low-density lipoprotein; IPP: intensive prevention programme; UC: usual care.
p < 0.05 vs. beginning of study.
#p < 0.05 vs. control cohort (usual care).
Comparison of risk factors during 12 months, intensive prevention program versus usual care.
| . | Beginning of study . | 12 months . |
|---|---|---|
| LDL cholesterol, mg/dl | ||
| MV ± SD | ||
| – IPP | 73.1 ± 26 | 67.6 ± 21*,# |
| – UC | 73.8 ± 26 | 78.4 ± 29 |
| Total cholesterol, mg/dl | ||
| MV ± SD | ||
| – IPP | 146.7 ± 30 | 143.2 ± 26# |
| – UC | 145.5 ± 28 | 153.3 ± 34* |
| Systolic blood pressure, mmHg | ||
| MV ± SD | ||
| – IPP | 133 ± 17 | 130 ± 15*,# |
| – UC | 132 ± 16 | 135 ± 18* |
| Diastolic blood pressure, mmHg | ||
| MV ± SD | ||
| – IPP | 80 ± 10 | 79 ± 9 |
| – UC | 81 ± 10 | 82 ± 12 |
| Body mass index, kg/m2 | ||
| MV ± SD | ||
| – IPP | 28.1 ± 3.8 | 28.0 ± 4.0 |
| – UC | 27.9 ± 3.7 | 28.3 ± 4.2* |
| HbA1c in diabetics, % | ||
| MV ± SD | ||
| – IPP | 6.7 ± 0.8 | 6.9 ± 0.7 |
| – UC | 6.7 ± 0.8 | 7.3 ± 1.8 |
| . | Beginning of study . | 12 months . |
|---|---|---|
| LDL cholesterol, mg/dl | ||
| MV ± SD | ||
| – IPP | 73.1 ± 26 | 67.6 ± 21*,# |
| – UC | 73.8 ± 26 | 78.4 ± 29 |
| Total cholesterol, mg/dl | ||
| MV ± SD | ||
| – IPP | 146.7 ± 30 | 143.2 ± 26# |
| – UC | 145.5 ± 28 | 153.3 ± 34* |
| Systolic blood pressure, mmHg | ||
| MV ± SD | ||
| – IPP | 133 ± 17 | 130 ± 15*,# |
| – UC | 132 ± 16 | 135 ± 18* |
| Diastolic blood pressure, mmHg | ||
| MV ± SD | ||
| – IPP | 80 ± 10 | 79 ± 9 |
| – UC | 81 ± 10 | 82 ± 12 |
| Body mass index, kg/m2 | ||
| MV ± SD | ||
| – IPP | 28.1 ± 3.8 | 28.0 ± 4.0 |
| – UC | 27.9 ± 3.7 | 28.3 ± 4.2* |
| HbA1c in diabetics, % | ||
| MV ± SD | ||
| – IPP | 6.7 ± 0.8 | 6.9 ± 0.7 |
| – UC | 6.7 ± 0.8 | 7.3 ± 1.8 |
Data are mean values ± standard deviations (MV ± SD).
LDL: low-density lipoprotein; IPP: intensive prevention programme; UC: usual care.
p < 0.05 vs. beginning of study.
#p < 0.05 vs. control cohort (usual care).
In a logistic regression model a significantly higher rate of the risk factor targets non-smoking, LDL cholesterol <70 mg/dl and systolic blood pressure <140 mmHg was observed in the IPP group compared with the usual care group after 12 months (Figure 2).
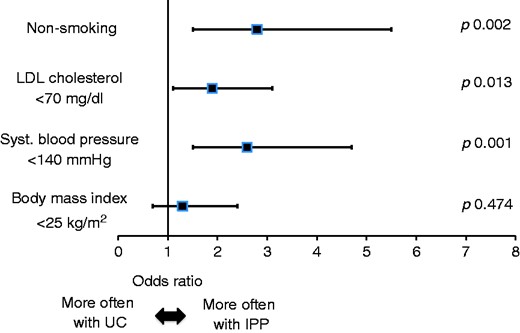
Logistic regression model on risk factor targets after 12 months in the intensive prevention programme versus usual care group.
LDL: low-density lipoprotein; UC: usual care; IPP: intensive prevention programme.
Physical activity was increased by 157% from beginning of study to 12-month visit in the IPP group (p < 0.01 vs. beginning of study and vs. usual care). In contrast, no significant changes were observed in the usual care group (+12% from beginning of study to 12 months, p = 0.22) (Supplementary Figure 2).
Telemetric control
Telemetric control of physical activity as part of the prevention programme was well accepted.
Step counters. Patients (79.5%) used step counters and documented number of steps online; 69.7% performed regular long-term documentation for longer than six months. Patients with long-term use of step counters increased their number of steps with growing time interval from MI, starting with 8673 ± 4118 steps/day at beginning of study and increasing the number of steps to 10,236 ± 5265 steps/day after 12 months (p < 0.001).
Activity trackers. The more complex system with activity trackers and direct online transmission of data, which was additionally offered to the patients since February 2015, was used by fewer study participants (31% of patients) than the step counters.
Medical therapy
A significant improvement of medical therapy was observed in the IPP group compared with the usual care group during the study course. This was most obvious regarding lipid lowering medication (LLM): 46 patients (33.3%) of the IPP group increased LLM, but only 17 patients (11.9%) of the usual care group (p < 0.01) did. Ten patients (7.2%) of the IPP group decreased LLM in contrast to 25 patients (17.5%) of the usual care group (p < 0.01).
Quality of life, depression
Self-rated quality of life (QoL) was improved in the IPP group compared with usual care, indicated by an increase from 76.4 ± 15 points at beginning of study to 78.2 ± 15 points after 12 months (p = 0.03) on the EQ-5D-5L visual analogue scale, while no change was observed in usual care (77.6 ± 13 points vs. 77.1 ± 14 points, p = 0.81). In the IPP group the PHQ-9 score on depression decreased from 4.3 ± 4.2 points at beginning of study to 3.6 ± 3.5 points after 12 months (p = 0.04), while an increase from 3.9 ± 3.5 points to 4.4 ± 3.5 points (p = 0.05) was observed in usual care.
Serious adverse events
Only few serious adverse events, such as deaths (IPP 0.7%, usual care 1.4%), reinfarctions (IPP 0.7%, usual care 1.4%) or unplanned revascularizations (IPP 5.1%, usual care 6.3%), occurred during the 12-month course of the randomized trial. The combined endpoint of death, stroke, reinfarction, unplanned revascularization or cardiovascular rehospitalization was slightly lower in the IPP group compared with usual care (13.8% vs. 18.9%) without significance (p = 0.25).
STEMI versus NSTEMI
IPP was associated with an improvement of risk factor control in both patients with STEMI (IPP Prevention Score 10.5 ± 1.9 points at beginning of study vs. 11.3 ± 2.3 points after 12 months, p < 0.01) and patients with NSTEMI (IPP Prevention Score 9.9 ± 2.5 points at beginning of study vs. 11.6 ± 2.2 points after 12 months, p < 0.01), with a stronger increase in patients with NSTEMI (p < 0.05). There were no significant differences in serious adverse events between the groups STEMI vs. NSTEMI (p > 0.05); however, this analysis was limited by case numbers.
Discussion
This randomized multicentre prevention trial revealed two major results:
A novel intensive prevention programme for patients after MI that included personal teachings and telemetric care and that was coordinated by a non-physician prevention assistant improved secondary prevention with significantly better risk factor control, better medical treatment and improvement of QoL.
In contrast, usual care was associated with a striking deterioration of risk factor control during long-term follow-up, although the patients of the usual care group were participants of a randomized trial with six-monthly study visits – a collective that is supposed to have better risk factor control compared with patients who refused study participation.
Observational studies, such as EUROASPIRE, demonstrated that secondary prevention in patients with CAD has not been improved during the last decades regarding various risk factors.2,3,18 Although medication adherence has a significant impact on prognosis, medical adherence of patients after MI is poor.19–21 Therefore it is obvious that effective long-term prevention programmes after MI are missing.1,10 Previous trials on prevention programmes reported controversial results and had arguable intervention duration, intensity and endpoints.4–9,22
In 2016 Piepoli et al. pointed out the need for adapted long-term prevention programmes with individualized interventions and usage of telecommunication technology in secondary prevention after acute MI.18 IPP represented such a programme, which clearly differed from previous studies on this topic:
A long-term programme with very intensive personal teachings by a prevention assistant was performed.
A prevention network, coordinated by a prevention assistant and including acute care hospitals, rehabilitation centres, general practitioners and cardiologists of the patient was implemented.
A focus was set on physical activity. In our view, implementation of physical activity in prevention programmes is crucial, as numerous studies, inter alia from our working group, demonstrated impressive beneficial effects in secondary prevention of CAD.23,24
Telemetric control of physical activity as part of a long-term prevention programme was included. Previous data on telemetric risk factor control in CAD patients are controversial and with small case numbers.25 To our knowledge this is the first completed study on telemetric risk factor control as part of a long-term clinical prevention programme after MI. The data show that simple step counters with manual online documentation are well accepted and used by more than three-quarters of all patients. The more complex activity trackers with online transmission were used by only one-third of all patients, probably due to the more difficult handling of these devices; however, these activity trackers can provide more objective, detailed, and meaningful information on activity.
The IPP study demonstrates beneficial effects of a prevention programme performed by well-educated and supervised prevention assistants at two specialized centres. As a consequence it seems reasonable to introduce prevention centres with specialized prevention assistants providing long-term care for CAD patients analogous to the concept of heart failure nursing. Obviously these strategies are more effective than the actual standard of care.
Regarding the effects of IPP on different risk factors, the modifications of body mass index after 12 months were smaller compared with effects on LDL cholesterol, systolic blood pressure or smoking. It appears that IPP strongly modified medical prevention, such as lipid or blood pressure lowering medication, and significantly improved smoking prevention, while the effects on body mass index were limited. It may be argued that body mass index is not an optimal parameter to measure prevention effects; but it is obvious that this parameter was difficult to modify by the prevention efforts of IPP.
Study limitations
The study was limited to interventionally treated patients; patients with CABG in the acute phase after MI were not included.
The rate of screened patients who refused study participation is a limitation and study bias, although higher rates of incompliant patients have been reported in other long-term prevention trials.8 It must be the aim for future prevention programmes to implement attractive prevention strategies (such as ‘easy to handle’ telemetric control) to reach all patients with MI.
The study sample size limited statistical analysis and detection of significance in the baseline numeric differences (smoking). Furthermore, the study was not powered for clinical events. However, it has been proven by numerous trials that better risk factor control and medical treatment is associated with a long-term reduction of major adverse events.1,11,13,14 Therefore the described effects by IPP should result in significant reductions of clinical events in further investigations with larger patient cohorts and longer follow-up.
Conclusions
This is the first randomized trial on a long-term prevention programme with very intensive personal teachings and telemetric risk factor control after MI. The prevention programme is based on a prevention network, coordinated by a non-physician prevention assistant.
This ‘intensive prevention programme concept’ was associated with significant improvements of long-term prevention and could serve as a model for better future prevention strategies.
Author contribution
HW and AF contributed equally. HW, AF, SM, JM, JS, HH, TH, AE and RH contributed to the conception or design of the work. All authors contributed to the acquisition, analysis or interpretation of the data. HW drafted the manuscript, all other authors critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Stiftung Bremer Herzen, Germany; Deutsche Herzstiftung e.V., Germany; Stiftung Versorgungsforschung der Arbeitsgemeinschaft Leitender Kardiologischer Krankenhausärzte (ALKK), Germany; Handelskrankenkasse Bremen, Germany and Stiftung Bremer Wertpapierbörse, Germany.
References
Author notes
These two authors contributed equally.




Comments