-
PDF
- Split View
-
Views
-
Cite
Cite
Tanja Zeller, Renate B Schnabel, Sebastian Appelbaum, Francisco Ojeda, Filip Berisha, Benedict Schulte-Steinberg, Burkhard-Ekkehart Brueckmann, Kari Kuulasmaa, Pekka Jousilahti, Stefan Blankenberg, Tarja Palosaari, Veikko Salomaa, Mahir Karakas, Low testosterone levels are predictive for incident atrial fibrillation and ischaemic stroke in men, but protective in women – results from the FINRISK study, European Journal of Preventive Cardiology, Volume 25, Issue 11, 1 July 2018, Pages 1133–1139, https://doi.org/10.1177/2047487318778346
Close - Share Icon Share
Abstract
Atrial fibrillation is the most common serious abnormal heart rhythm, and a frequent cause of ischaemic stroke. Recent experimental studies, mainly in orchiectomised rats, report a relationship between sex hormones and atrial electrophysiology and electroanatomy. We aimed to evaluate whether low testosterone levels are predictive for atrial fibrillation and/or ischaemic stroke in men and women.
The serum total testosterone levels were measured at baseline in a population cohort of 7892 subjects (3876 male, 4016 female), aged 25–74 years, using a commercially available immunoassay. The main outcome measure was atrial fibrillation or ischaemic stroke, whichever came first.
During a median follow-up of 13.8 years, a total of 629 subjects (8.0%) suffered from incident atrial fibrillation (n = 426) and/or ischemic stroke (n = 276). Cox regression analyses, adjusted for age (used as time-scale), geographical region, total cholesterol (log), high-density lipoprotein-cholesterol (log), hypertension medication, known diabetes, smoking status, waist-hip-ratio, and time of blood drawn, documented differential predictive value of low sex-specific testosterone levels for atrial fibrillation and/or ischaemic stroke, in men and in women: Increasing levels were associated with lower risk in men (hazard ratio per one nmol/l increase 0.98 (95% confidence interval 0.93–1.00); p = 0.049). On the other hand, increasing testosterone levels were associated with higher risk in women (hazard ratio per one nmol/l increase 1.17 (95% confidence interval 1.02–1.36); p = 0.031).
Our study indicates that low testosterone levels are associated with increased risk of future atrial fibrillation and/or ischaemic stroke in men, while they are protective in women.
Introduction
The incidence and prevalence of atrial fibrillation (AF) in the community have increased dramatically in the western world in recent decades.1,2 The rhythm disorder ranks among the most frequent underlying or contributory causes of death.3 AF represents a major public health burden with high comorbidity, increased mortality risk, and soaring healthcare costs.4–8 The most life-threatening sequelae of AF are thromboembolic events, in particular ischaemic stroke (IST).9,10
The risk of incident AF is higher in elderly men than in women, indicating that risk factors for AF might be sex-specific.11 Accordingly, a relationship between sex hormones, atrial electrophysiology and electroanatomy,12,13 as well as to cardiovascular outcomes and mortality14 has been shown. Studies found that high levels of testosterone, the primary male sex hormone, were effective in the prevention and reduction of atrial arrhythmias,13,15 whereas older men with decreased testosterone were at increased risk of AF.14 However, only little is known on the effect of testosterone levels in women.16
In this study, we aimed to evaluate the predictive role of serum testosterone levels for incident AF and/or IST in men and women in a large population-based European cohort.
Methods
All analyses and biomarker measurements presented were performed in the framework of the BiomarCaRE consortium.17
Study population
The present study is based on individuals from the FINRISK97 cohort aged 25–74 years drawn from the national Finnish population register in 1997. The design of the FINRISK97 study has been published elsewhere.18 This prospective population-based study was carried out in five districts; North Karelia, Northern Savo (former Kuopio), Southwestern Finland, Oulu Province, and the region of Helsinki and Vantaa. Altogether 11,500 individuals were invited, and 8444 (73%) participated in the clinical examination. During the follow-up period of up to 15 years, the follow-up rate was 100% for the participants who continued living in Finland. Those who had permanently moved abroad (0.5% of the participants prior to 31 December 2011) were lost to follow-up. All individuals enrolled in the study received a physical examination and a self-administered questionnaire and a blood sample was drawn. Prior to drawing the blood samples, the individuals were asked to have a four-hour fasting period, avoiding heavy meals earlier during the day. The median fasting time was five hours with an interquartile range of 3–7 h. The blood samples were stored under standardised conditions at −80℃. For the present article, we excluded women who were pregnant at the time of the clinical examination, individuals on testosterone-supplementation therapy, and individuals with prevalent cancer, AF or IST. Final analyses were thus based on 7892 subjects, (49.1% men and 50.9% women) individuals. The Ethics Committee of the National Public Health Institute approved the study, which followed the Declaration of Helsinki. All subjects gave written informed consent.
Outcome information
The National Hospital Discharge Register and the National Causes of Death Register were used to identify incident AF and IST during a follow-up of up to 15 years. The use of Finnish national healthcare registries for identifying disease outcomes has recently been demonstrated.19,20
Laboratory methods
Blood samples were stored under standardised conditions at –80℃. Routine laboratory parameters were measured at the Disease Risk Unit in the National Institute for Health and Welfare, Helsinki, Finland. The estimated glomerular filtration rate (eGFR) was calculated using the CKD-Epi equation.21 The measurement of total testosterone levels was performed at the BiomarCaRE/MORGAM Laboratory (University Heart Center Hamburg, Germany), using a chemiluminescent microparticle immunoassay (CMIA) (Abbott ARCHITECT 2nd Generation Testosterone; Abbott Diagnostics). The assay range was 0.45–35 nmol/l, the intra-assay coefficient of variation (CV) was 3.57%, the inter-assay CV was 8.83%.
Statistical methods
Missing values (n = 875) were dealt using multiple imputation via chained equations.22 Baseline characteristics are presented as percentages for dichotomous variables, and as quartiles for continuous variables. Correlation analysis was performed using Pearson correlation coefficients. Additionally, age-adjusted Pearson correlations were calculated. Because of a skewed distribution total cholesterol values, high-density lipoprotein (HDL)-cholesterol values and testosterone values in men were log-transformed for analyses.
Age-adjusted Kaplan-Meier curves for incident AF and/or IST were produced, using categorised (by sex-specific quartiles) testosterone concentrations. To examine the association of testosterone with AF and/or IST, Cox regression models with age as the timescale were used. Each sex was considered separately due to the different shape of the testosterone distributions in men and women. Cox regression was performed with testosterone as a continuous variable. Continuous testosterone was log-transformed for women and left untransformed for men. Two different adjustments were used. Model 1 used age as the timescale, and adjusted for geographical region of Finland (east, west). In model 2 additional adjustment was done for log-transformed total cholesterol, log-transformed HDL-cholesterol, hypertension medication, diabetes, smoking status, waist-hip-ratio, and time of blood draw, since testosterone values undergo a circadian rhythm.
R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses. All tests were two-tailed, and p < 0.05 was considered statistically significant.
Results
The baseline characteristics of the study participants are presented in Table 1. The median age of the participants was 49.2 years for men and 47.2 years for women. Men in general had a more adverse cardiovascular risk profile than women. Testosterone levels were clearly higher in male subjects (median 17.10 versus 1.15 nmol/l; p < 0.001).
| . | Men . | Women . |
|---|---|---|
| n | 3876 | 4016 |
| Age (years), SD | 49.2 (37.7; 60.7) | 47.2 (36.5; 57.6) |
| BMI (kg/m2), SD | 26.6 (24.3; 29.1) | 25.5 (22.7; 29.1) |
| Waist-hip-ratio, SD | 0.93 (0.88; 0.97) | 0.79 (0.75; 0.84) |
| Diabetes | 5.7 | 5.2 |
| Current smoker (%) | 26.3 | 17.4 |
| Hypertension (%) | 17.6 | 14.3 |
| HDL-C (mmol/l), SD | 1.23 (1.04; 1.43) | 1.51 (1.28; 1.75) |
| Total-cholesterol (mmol/l), SD | 5.5 (4.8; 6.2) | 5.4 (4.7; 6.2) |
| Systolic blood pressure (mm Hg), SD | 137 (126; 152) | 130 (118; 145) |
| Testosterone (nmol/l), IQR | 17.10 (12.87; 22.04) | 1.15 (0.87; 1.56) |
| . | Men . | Women . |
|---|---|---|
| n | 3876 | 4016 |
| Age (years), SD | 49.2 (37.7; 60.7) | 47.2 (36.5; 57.6) |
| BMI (kg/m2), SD | 26.6 (24.3; 29.1) | 25.5 (22.7; 29.1) |
| Waist-hip-ratio, SD | 0.93 (0.88; 0.97) | 0.79 (0.75; 0.84) |
| Diabetes | 5.7 | 5.2 |
| Current smoker (%) | 26.3 | 17.4 |
| Hypertension (%) | 17.6 | 14.3 |
| HDL-C (mmol/l), SD | 1.23 (1.04; 1.43) | 1.51 (1.28; 1.75) |
| Total-cholesterol (mmol/l), SD | 5.5 (4.8; 6.2) | 5.4 (4.7; 6.2) |
| Systolic blood pressure (mm Hg), SD | 137 (126; 152) | 130 (118; 145) |
| Testosterone (nmol/l), IQR | 17.10 (12.87; 22.04) | 1.15 (0.87; 1.56) |
BMI: body mass index; HDL-C: high-density lipoprotein-cholesterol; SD: standard deviation.
For continuous variables, median (25th percentile, 75th percentile) are shown. For binary variables percentage is given. Imputation was performed on 875 data sets.
| . | Men . | Women . |
|---|---|---|
| n | 3876 | 4016 |
| Age (years), SD | 49.2 (37.7; 60.7) | 47.2 (36.5; 57.6) |
| BMI (kg/m2), SD | 26.6 (24.3; 29.1) | 25.5 (22.7; 29.1) |
| Waist-hip-ratio, SD | 0.93 (0.88; 0.97) | 0.79 (0.75; 0.84) |
| Diabetes | 5.7 | 5.2 |
| Current smoker (%) | 26.3 | 17.4 |
| Hypertension (%) | 17.6 | 14.3 |
| HDL-C (mmol/l), SD | 1.23 (1.04; 1.43) | 1.51 (1.28; 1.75) |
| Total-cholesterol (mmol/l), SD | 5.5 (4.8; 6.2) | 5.4 (4.7; 6.2) |
| Systolic blood pressure (mm Hg), SD | 137 (126; 152) | 130 (118; 145) |
| Testosterone (nmol/l), IQR | 17.10 (12.87; 22.04) | 1.15 (0.87; 1.56) |
| . | Men . | Women . |
|---|---|---|
| n | 3876 | 4016 |
| Age (years), SD | 49.2 (37.7; 60.7) | 47.2 (36.5; 57.6) |
| BMI (kg/m2), SD | 26.6 (24.3; 29.1) | 25.5 (22.7; 29.1) |
| Waist-hip-ratio, SD | 0.93 (0.88; 0.97) | 0.79 (0.75; 0.84) |
| Diabetes | 5.7 | 5.2 |
| Current smoker (%) | 26.3 | 17.4 |
| Hypertension (%) | 17.6 | 14.3 |
| HDL-C (mmol/l), SD | 1.23 (1.04; 1.43) | 1.51 (1.28; 1.75) |
| Total-cholesterol (mmol/l), SD | 5.5 (4.8; 6.2) | 5.4 (4.7; 6.2) |
| Systolic blood pressure (mm Hg), SD | 137 (126; 152) | 130 (118; 145) |
| Testosterone (nmol/l), IQR | 17.10 (12.87; 22.04) | 1.15 (0.87; 1.56) |
BMI: body mass index; HDL-C: high-density lipoprotein-cholesterol; SD: standard deviation.
For continuous variables, median (25th percentile, 75th percentile) are shown. For binary variables percentage is given. Imputation was performed on 875 data sets.
In order to assess the correlation of testosterone levels with clinical variables, Pearson correlation coefficients were calculated (Table 2). Age-adjusted Pearson analyses revealed statistically significant correlations of testosterone levels with HDL-cholesterol levels (R = 0.22, p < 0.001), body-mass-index (R = −0.23; p < 0.001), waist-to-hip-ratio R = −0.22; p < 0.001), and time of blood draw (R = −0.10; p < 0.001) in men. In women, no significant correlations were found, although correlations with total cholesterol (R = −0.03; p = 0.065) and time of blood draw (R = −0.03; p = 0.055) were borderline non-significant.
Age-adjusted Pearson correlation coefficients of testosterone levels with clinical variables.
| . | Men (R, p-value) . | Women (R, p-value) . |
|---|---|---|
| Time of day of the blood draw | −0.10 <0.001 | −0.03 0.055 |
| Smoking | 0.09 <0.001 | 0.009 0.97 |
| Total cholesterol | 0.009 0.80 | −0.03 0.065 |
| HDL-C | 0.22 <0.001 | −0.03 0.10 |
| Systolic blood pressure | −0.05 0.004 | 0.01 0.51 |
| eGFR | 0.01 0.58 | −0.02 0.20 |
| BMI | −0.23 <0.001 | 0.01 0.47 |
| WHR | −0.22 <0.001 | 0.01 0.68 |
| . | Men (R, p-value) . | Women (R, p-value) . |
|---|---|---|
| Time of day of the blood draw | −0.10 <0.001 | −0.03 0.055 |
| Smoking | 0.09 <0.001 | 0.009 0.97 |
| Total cholesterol | 0.009 0.80 | −0.03 0.065 |
| HDL-C | 0.22 <0.001 | −0.03 0.10 |
| Systolic blood pressure | −0.05 0.004 | 0.01 0.51 |
| eGFR | 0.01 0.58 | −0.02 0.20 |
| BMI | −0.23 <0.001 | 0.01 0.47 |
| WHR | −0.22 <0.001 | 0.01 0.68 |
BMI: body mass index; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; R: correlation coefficient; WHR: waist-to-hip ratio.
Age-adjusted Pearson correlation coefficients of testosterone levels with clinical variables.
| . | Men (R, p-value) . | Women (R, p-value) . |
|---|---|---|
| Time of day of the blood draw | −0.10 <0.001 | −0.03 0.055 |
| Smoking | 0.09 <0.001 | 0.009 0.97 |
| Total cholesterol | 0.009 0.80 | −0.03 0.065 |
| HDL-C | 0.22 <0.001 | −0.03 0.10 |
| Systolic blood pressure | −0.05 0.004 | 0.01 0.51 |
| eGFR | 0.01 0.58 | −0.02 0.20 |
| BMI | −0.23 <0.001 | 0.01 0.47 |
| WHR | −0.22 <0.001 | 0.01 0.68 |
| . | Men (R, p-value) . | Women (R, p-value) . |
|---|---|---|
| Time of day of the blood draw | −0.10 <0.001 | −0.03 0.055 |
| Smoking | 0.09 <0.001 | 0.009 0.97 |
| Total cholesterol | 0.009 0.80 | −0.03 0.065 |
| HDL-C | 0.22 <0.001 | −0.03 0.10 |
| Systolic blood pressure | −0.05 0.004 | 0.01 0.51 |
| eGFR | 0.01 0.58 | −0.02 0.20 |
| BMI | −0.23 <0.001 | 0.01 0.47 |
| WHR | −0.22 <0.001 | 0.01 0.68 |
BMI: body mass index; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; R: correlation coefficient; WHR: waist-to-hip ratio.
During a median follow-up of 13.8 years, a total of 629 subjects (8.0%) suffered from incident AF (n = 426) and/or IST (n = 276). In men, Kaplan-Meier analyses showed a strong association of low testosterone levels with incident AF and/or IST (Figure 1), while women at increased testosterone quartiles were at higher risk for AF and/or IST. In accordance, fully adjusted Cox regression analyses in men indicated higher risk of future AF and IST in those with low testosterone levels (hazard ratio (HR) 0.98, 95% confidence interval (CI) 0.97–1.00; p = 0.049 per one unit increase) (Table 3). In contrast, in women, fully adjusted Cox regression analysis, indicated increased risk of future AF and IST with increasing testosterone levels (HR 1.17, 95% CI 1.02–1.36, p = 0.031 per one nmol/l increase). Supplementary Material Figures 1 and 2 show Kaplan-Meier analyses for each endpoint separately. Adjustment to body mass index instead of waist-to-hip-ratio and log-transformed systolic blood pressure instead of hypertensive medication did not alter the Cox regression results. Comparable to the analyses of the combined endpoint, crude Cox regression analyses proofed significant association of testosterone levels for the separate endpoints, with AF in men (HR 0.98, 95% CI 0.96–0.99, p for trend 0.008), with IST in men (HR 0.97, 95% CI 0.95–0.99, p for trend 0.003), with IST in women (HR 1.33, 95% CI 1.09–1.62, p for trend 0.005), and with AF in women (HR 1.22, 95% CI 1.04–1.42, p for trend 0.014).
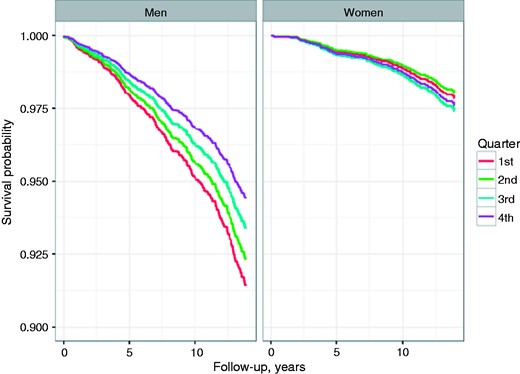
Age-adjusted Kaplan-Meier-Curves for freedom from atrial fibrillation (AF) and/or ischaemic stroke (IST) during follow-up, according to testosterone categories. First quartile representing lowest and fourth quartile highest testosterone levels.
Association (hazard ratio (HR), 95% confidence interval (CI)) of baseline serum testosterone levels with incident atrial fibrillation (AF) and/or ischaemic stroke (IST) during follow-up.
| . | HR per one nmol/l increase (95% CI) . | p-Value . |
|---|---|---|
| Men | ||
| Model 1 | 0.97 (0.96–0.99) | <0.0001 |
| Model 2 | 0.98 (0.97–1.00) | 0.049 |
| Women | ||
| Model 1 | 1.28 (1.12–1.46) | <0.0001 |
| Model 2 | 1.17 (1.02–1.36) | 0.031 |
| . | HR per one nmol/l increase (95% CI) . | p-Value . |
|---|---|---|
| Men | ||
| Model 1 | 0.97 (0.96–0.99) | <0.0001 |
| Model 2 | 0.98 (0.97–1.00) | 0.049 |
| Women | ||
| Model 1 | 1.28 (1.12–1.46) | <0.0001 |
| Model 2 | 1.17 (1.02–1.36) | 0.031 |
HDL: high-density lipoprotein; SD: standard deviation.
Model 1: age was used as time-scale, adjusted for geographical region. Model 2: additionally, adjusted for total cholesterol (log), HDL-cholesterol (log), systolic blood pressure (log), hypertension medication, known diabetes, smoking status, waist-hip-ratio, and time of day of the blood draw.
Association (hazard ratio (HR), 95% confidence interval (CI)) of baseline serum testosterone levels with incident atrial fibrillation (AF) and/or ischaemic stroke (IST) during follow-up.
| . | HR per one nmol/l increase (95% CI) . | p-Value . |
|---|---|---|
| Men | ||
| Model 1 | 0.97 (0.96–0.99) | <0.0001 |
| Model 2 | 0.98 (0.97–1.00) | 0.049 |
| Women | ||
| Model 1 | 1.28 (1.12–1.46) | <0.0001 |
| Model 2 | 1.17 (1.02–1.36) | 0.031 |
| . | HR per one nmol/l increase (95% CI) . | p-Value . |
|---|---|---|
| Men | ||
| Model 1 | 0.97 (0.96–0.99) | <0.0001 |
| Model 2 | 0.98 (0.97–1.00) | 0.049 |
| Women | ||
| Model 1 | 1.28 (1.12–1.46) | <0.0001 |
| Model 2 | 1.17 (1.02–1.36) | 0.031 |
HDL: high-density lipoprotein; SD: standard deviation.
Model 1: age was used as time-scale, adjusted for geographical region. Model 2: additionally, adjusted for total cholesterol (log), HDL-cholesterol (log), systolic blood pressure (log), hypertension medication, known diabetes, smoking status, waist-hip-ratio, and time of day of the blood draw.
Discussion
In this study, we evaluated the predictive value of serum testosterone levels for the incidence of AF and IST in men and women.
Our data of a general population in a European country indicates an association between low testosterone levels and future risk of AF and IST in men, while low values seem protective in women. Median testosterone levels were 17.10 (12.87; 22.04) nmol/l in men, and 1.15 (0.87; 1.56) nmol/l in women. This is in line with normal values which range between 9–38 nmol/l in men, and 0.52–2.4 nmol/l in women. In women the increase in risk per one unit SD of testosterone is bigger, since the range of testosterone levels in women is clearly lower.
AF is the most common serious abnormal heart rhythm, and a frequent cause of IST. This relationship was illustrated in two major trials comparing ischaemic brain events in patients with AF and those with carotid disease:23 within the NASCET and the SPAF trials, the ratio of hemispheric events to retinal events was 25:1 with AF, compared with 2:1 with carotid disease. Further evidence was provided recently by the landmark trial CRYSTAL-AF, which by intra-cardiac ECG monitoring over 36 months revealed, that a large proportion of subjects deemed as cryptogenic stroke, indeed had underlying asymptomatic intermittent AF.24
Moreover, as evidenced by the Framingham and the Copenhagen stroke studies, IST occurring with AF is more likely to be fatal or more severe than IST occurring in the absence of AF, even after adjustment for the advanced age of patients with AF-related stroke.25,26
Earlier studies have demonstrated that not only risk factors like age, diabetes, hypertension and obesity, but also cardiovascular disease including alterations in cardiac structure and function consistently predispose to AF.3,4 The pathway from altered circulating levels of testosterone to AF seems heterogenous. Various experimental studies suggest, that age and systemic hormones alter electrophysiology and electroanatomy in the atrium.12,13,27,28 In a human study involving 13 patients above the age of 60 years, 13 patients aged 30–60 years, and 15 patients below the age of 30 years, aging was associated with an increase in atrial refractoriness, prolonged conduction time along the distal coronary sinus, increased p-wave duration, and an increase in the maximum corrected sinus node recovery time, all mechanisms which are postulated to contribute to AF susceptibility.27 Other studies proved that testosterone is a potent inhibitor of L-type calcium channels, and that testosterone blocks the effects of acetylcholine on the atria, and thereby may reduce excitability and automaticity of the atrium – two AF-inducing pathomechanisms.28 Another electrophysiological study in the rat model showed, that the immunoreactive protein levels of ryanodine receptor type 2 (RyR2) and sodium-calcium exchanger significantly increased in orchiectomised male rats as compared with sham-operated male rats, and orchiectomised male rats with administration of testosterone, without alterations in the level of FK506-binding protein (FKBP12.6), a modulator of the calcium-release ryanodine receptors (RyRs).12 This suggests that deficiency of testosterone is arrhythmogenic in the atrium through less binding of FKBP12.6 to RyR2, which probably induces feasible calcium leakage from the sarcoendoplasmic reticulum.13,29–31 Interestingly, and in line with our results, this study in the rat model also proved a differential effect of testosterone deficiency in orchiectomised male and ovariectomised female rats.12
The finding of moderate but statistically significant correlations of testosterone levels with body mass index and waist-to-hip-ratio in men is in line with earlier data from cross-sectional studies.32 We add to the scientific state of knowledge, by demonstrating that this correlation is sex-specific, and not relevant in women. The negative correlation between body mass index/waist-to-hip-ratio and circulating testosterone levels in men seems to be explained by enhanced conversion of androstenedione to oestrogens in obese males by aromatization, which occurs due to adipose tissue-driven elevated aromatase levels.33,34 Moreover, studies in rodents and humans have documented that leptin, produced by adipose tissue, inhibits testosterone secretion from the Leydig cells.35,36
Strengths and limitations
This study represents the largest prospective study of its kind and, unlike most others before, also included women. Our study has some limitations that need to be addressed. First, although we adjusted for major risk factors for AF like sex, age, hypertension and diabetes, no further information on additional risk factors of atrial fibrillation such as thyroid function and left-ventricular hypertrophy were available that could have potentially influenced the findings of the study. Second, no serial measurements were available and therefore, we could not explore the impact of changes on testosterone levels towards future disease development. Third, we are not able to comment on whether intra-individual increasing (e.g. under supplementation therapy) or decreasing testosterone levels (e.g. under statin intake) would impact future risk of AF and IST. Fourth, testosterone exerts diurnal variation and our samples have been drawn throughout the day. Accordingly, Cox regression analyses were adjusted for time of blood drawn in the advanced model (although correlation with testosterone levels was negligible; Table 3). Fifth, like most recent publications, we did not measure levels of sex hormone-binding globulin (SHBG) and free testosterone. Usually, bioavailable and free testosterone levels parallel total testosterone levels. However, a number of rare conditions are known to increase or decrease the SHBG levels, which may cause total testosterone levels to change without necessarily influencing the bioavailable or free testosterone levels, or vice versa. Measurement of total testosterone is in line with clinical reality and respective recommendations of the Food and Drug Administration (FDA) which, in its latest statement, urges measurement of total testosterone only. Finally, samples had been stored for almost 20 years and degradation processes, which would affect both individuals with and without events, cannot be excluded.
Conclusion
In our large-scale population-based study, in line with previous experimental studies, low testosterone concentrations are associated with increased risk of future AF and/or IST in men, while they are protective in women.
Given this differential predictive value, AF and IST should be implemented as secondary endpoints in ongoing and upcoming clinical trials investigating the effects of testosterone supplementation.
Translational perspective
Testosterone deficiency is a wide-spread entity in men. Low testosterone concentrations seem to be associated with increased risk of future AF and/or IST in men, while they appear protective in women. The efficacy of testosterone supplementation to lower rates of AF and IST in men should be investigated. Testosterone measurements in patients with AF and IST might add to clinical information.
Author contribution
TZ, RBS, PJ, VS, KK, MK contributed to the conception or design of the work. TZ, SeA, FO, FB, BSS, BB, SB, TP, VS, KK, MK contributed to the acquisition, analysis or interpretation of the work. TZ, VS, KK, MK drafted the manuscript. TZ, RBS, SeA, FO, FB, BSS, BB, SB, TP, PJ, VS, KK and MK critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy. MK and TZ had full access to all data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been supported by the European Union's Seventh Framework Program (FP7/2007-2013) under grant agreement No. HEALTH-F2-2011-278913 (BiomarCaRE), and the European Research Area Network on Cardiovascular Diseases (ERA-CVD) that has been granted for funding through the current EU Framework Programme for Research and Innovation ‘Horizon 2020’. VS has been supported by the Finnish Foundation for Cardiovascular Research.




Comments