-
PDF
- Split View
-
Views
-
Cite
Cite
Giuseppe Ambrosio, Alessandro Mugelli, José Lopez-Sendón, Juan Tamargo, John Camm, Management of stable angina: A commentary on the European Society of Cardiology guidelines, European Journal of Preventive Cardiology, Volume 23, Issue 13, 1 September 2016, Pages 1401–1412, https://doi.org/10.1177/2047487316648475
Close - Share Icon Share
Abstract
In 2013 the European Society of Cardiology (ESC) released new guidelines on the management of stable coronary artery disease. These guidelines update and replace the previous ESC guidelines on the management of stable angina pectoris, issued in 2006. There are several new aspects in the 2013 ESC guidelines compared with the 2006 version. This opinion paper provides an in-depth interpretation of the ESC guidelines with regard to these issues, to help physicians in making evidence-based therapeutic choices in their routine clinical practice. The first new element is the definition of stable coronary artery disease itself, which has now broadened from a ‘simple’ symptom, angina pectoris, to a more complex disease that can even be asymptomatic. In the first-line setting, the major changes in the new guidelines are the upgrading of calcium channel blockers, the distinction between dihydropyridines and non-dihydropyridine calcium channel blockers, and the presence of important statements regarding the combination of calcium channel blockers with beta-blockers. In the second-line setting, the 2013 ESC guidelines recommend the addition of long-acting nitrates, ivabradine, nicorandil or ranolazine to first-line agents. Trimetazidine may also be considered. However, no clear distinction is made among different second-line drugs, despite different quality of evidence in favour of these agents. For example, the use of ranolazine is supported by strong and recent evidence, while data supporting the use of the traditional agents appear relatively scanty.
Introduction
In 2013 the European Society of Cardiology (ESC) released new guidelines on the management of stable coronary artery disease (SCAD).1 (Please note that the abbreviation ‘SCAD’ has also been used to denote spontaneous coronary artery dissection.) These guidelines update and replace the previous ESC guidelines on the management of stable angina pectoris.2 There are several new aspects in the 2013 ESC guidelines compared with the 2006 version.
Three main topics in these new guidelines warrant further consideration: the new definition of SCAD and its implications for clinical practice, first-line therapy for symptom management and second-line therapy.
The aim of this opinion paper is to contribute to an in-depth interpretation of the ESC guidelines with regard to these three issues, to help physicians in making evidence-based therapeutic choices in their routine clinical practice.
New definition of SCAD and implications for clinical practice
A most important difference between new and previous guidelines is the concept of SCAD. In fact, the definition of the condition has recently evolved, moving from the concept of stable angina as a clinical condition to the broader concept of SCAD, which encompasses both the clinical condition and the pathogenetic mechanisms underlying ischaemic heart disease. The 2013 ESC guidelines define SCAD as follows:
This new definition of SCAD represents a major paradigm shift. The focus has moved from the symptom ‘angina pectoris’ to the pathophysiology of ischaemia in SCAD, that is, a mismatch between oxygen supply and demand, a condition that may also be asymptomatic, particularly during the stable phases that follow an acute coronary syndrome (ACS).1 A number of different mechanisms may limit coronary blood flow such as fixed atheromatous stenoses, raised left ventricular end-diastolic pressure, limited coronary vasodilation capacity, etc., all of which may be susceptible to anti-anginal therapies.Stable coronary artery disease is generally characterised by episodes of reversible myocardial demand/supply mismatch, related to ischaemia or hypoxia, which are usually inducible by exercise, emotion or other stress and reproducible – but, which may also be occurring spontaneously. Such episodes of ischaemia/hypoxia are commonly associated with transient chest discomfort (angina pectoris).1
Although previous and current guidelines identify the prevention of cardiovascular events and symptom relief as the two aims of treatment, by focusing on SCAD rather than on the ‘symptom’ of angina pectoris the 2013 ESC guidelines assume that in patients with chronic ischaemic heart disease myocardial ischaemia occurs in a significant number of patients over a period of time, and emphasise that SCAD patients should continue treatment regardless of the presence of symptoms, in order to offer optimal cardiovascular prevention.
In this respect, the new ESC guidelines are similar to the latest US (2012 American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons) guidelines for the diagnosis and management of patients with stable ischaemic heart disease (SIHD), which also specifically address the management of asymptomatic patients with SIHD.3
This new definition probably has also an impact on the epidemiological description of the condition. For example, the prevalence of disease appears greater in current guidelines compared to previous ones, especially in younger patients (e.g. 4–7% in men aged 45–64 years in current guidelines compared with 2–5% in the previous guidelines).1,2 These changes in the epidemiological data reflect the broader definition of disease embraced by the 2013 guidelines, but may be also attributed to the longer life expectancy of the population; people live longer thus increasing the prevalence of cardiovascular diseases (CVDs).
Current guidelines do not make any specific reference to the prevalence of persistent angina in spite of revascularisation, or to that of asymptomatic (i.e. ‘silent’) ischaemia post-revascularisation. Yet, the persistence of angina after revascularisation is not an infrequent event; with approximately 20–35% of patients reporting anginal symptoms 1 year after revascularisation,4–8 and the prevalence of angina can be as high as 60% in revascularised patients with diabetes mellitus (DM).9 As for the prevalence of ‘silent’ ischaemia, it varies widely, ranging from approximately equal to 2.5–45%, depending on the studied population and the criteria used for defining silent ischaemia.10,11 In general, asymptomatic coronary artery disease is more prevalent among patients with DM.10,11
It is noteworthy that current guidelines mention the entity of microvascular angina, that is, angina due to microvascular disease in patients who have objective myocardial ischaemia but coronary angiograms without fixed or dynamic obstructions in the epicardial coronary arteries.1 This is a common condition in clinical practice, particularly in women,12 but information on its specific management is limited.13
Diagnosis of SCAD
Other novel aspects of the 2013 ESC guidelines include a recommendation to base the diagnostic algorithm on pre-test probability, and the definition and methods for defining high-risk patients who may benefit from revascularisation.1 In particular, the guidelines recommend a stepwise approach for decision-making, which begins with a standard assessment of the probability that SCAD is present in a given patient (step 1). Step 1 is followed by non-invasive testing aimed at establishing the diagnosis of SCAD or non-obstructive atherosclerosis in patients with an intermediate probability of disease (step 2). No further investigations are recommended in individuals with a very low probability of coronary artery disease; and in very high probability cases the diagnosis is also accepted without further testing. This approach represents a clear shift in the diagnostic process and may be confusing, but it is time-saving and safe, the majority of cases being in the group of patients in whom further testing is recommended. Once the diagnosis of SCAD has been made, optimal medical therapy (OMT) is instituted and stratification for risk of subsequent events is carried out (step 3), in order to select patients who may be considered as candidates for invasive investigation and revascularisation. For risk stratification, several scores are recommended, but none has been universally accepted in clinical practice, and this issue is an excellent opportunity for future clinical research in this population.
Treatment of SCAD
Current ESC guidelines state that the aim of the management of SCAD is the reduction of symptoms and the prevention of cardiovascular events. The management of SCAD encompasses lifestyle modification, patient education, control of coronary artery disease risk factors, pharmacological therapy and revascularisation in selected cases.
Lifestyle
Lifestyle modifications should be viewed in a positive, proactive, rather than a negative way, by focusing on the benefits gained from a correct behaviour (i.e. abstinence from smoking, diet and exercise) rather than on risks from a negative behaviour (i.e. continuing to smoke, or persisting with a sedentary lifestyle). In more detail, the benefits of smoking cessation have been extensively reported,14 and quitting smoking is potentially the most effective of all preventive measures, as it is associated with a 36% reduction in mortality after myocardial infarction.14 Thus, smoking status should be assessed systematically and all smokers should be offered assistance to stop.15 As for diet, it is widely accepted that a healthy diet reduces the risk of CVD. Energy intake should be limited to the amount of energy needed to maintain (or obtain) a healthy weight (body mass index 25 kg/m2). In general, when following the rules for a healthy diet, no dietary supplements are needed. The largest study ever conducted with a so-called ‘Mediterranean’ diet16 showed a reduction in the incidence of major cardiovascular events in patients at high risk of cardiovascular events but without prior CVD.17 Finally, weight reduction in overweight and obese people by exercise is recommended in order to achieve favourable effects on blood pressure, dyslipidaemia and glucose metabolism.15 In particular, SCAD patients should undergo a gradual programme of aerobic exercise three or more times a week, for at least 30 minutes per session.1
Medical therapy
Regardless of whether patients are revascularised or not, guidelines recommend that OMT should be instituted. This is an overarching concept, which is also emphasised in the current American Heart Association/American College of Cardiology guidelines. However, in spite of this general agreement, what precisely constitutes OMT is less well defined,1 and a specific definition for OMT is not clearly provided. This probably reflects a general lack of consensus on what should constitute OMT, and it remains an open issue in the management of SCAD without a universally accepted definition.4,9,18
As for secondary prevention, medical therapy includes statins and aspirin in all cases without contraindications. Anti-platelet drugs are also recommended for prespecified periods of time after ACSs and coronary stenting, but the necessary duration of treatment is not known because of the balance between adverse bleeding events and improved survival. Anticoagulants may be considered in selected cases. There is an ongoing debate about the optimal cholesterol levels and the selection of statins. The results of new trials including the IMPROVE-IT,19 PRA-2P,20 ATLAS,21 PEGASUS,22 and the approval for use by the regulatory agencies may change the current guideline recommendations for the use of ezetimibe, vorapaxar, rivaroxaban and new anti-platelet drugs.
The goal of OMT should be to control ischaemia, and this aim has traditionally involved lessening oxygen demand by using calcium channel blockers (CCBs), which decrease afterload, reducing heart rate with beta-blockers and, more recently, ivabradine, decreasing contractility through beta-blockers and reducing preload by nitrates (Figure 1). Nitrates lose their activity if regularly taken over a prolonged period without a nitrate-free interval of about 8–10 hours. On the other hand, oxygen supply can be increased by avoiding vasospasm using vasodilators (e.g. long-acting nitrates), by preventing thrombus formation with anti-platelet or anticoagulant drugs, and by counteracting atherosclerotic coronary artery disease progression through statins.
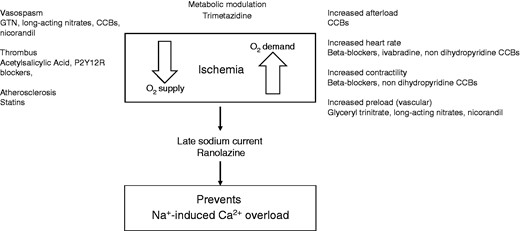
Supply and demand mismatch, and therapeutic targets in angina (elaborated from Montalescot et al.).1
A recently introduced agent, ranolazine, exerts its anti-ischaemic and anti-anginal effects through a mechanism of action that does not depend on changes in haemodynamic parameters; instead ranolazine restores the balance between myocardial oxygen demand and supply by preventing the pathological persistent opening of the late INa channels, with subsequent sodium and sodium-induced calcium overload responsible for the increase in diastolic myocardial tension.1 Formerly, ranolazine was thought to be effective against angina by inhibiting fatty acid oxidase activity. While this metabolic effect may be caused by ranolazine, it occurs only at concentrations above those seen in clinical practice. The guidelines also mention nicorandil, a nitrate derivative that stimulates ATP-sensitive potassium channels and trimetazidine, an anti-ischaemic metabolic modulator.1 The use of allopurinol in this setting has also been suggested due to its anti-oxidant effect.1
First-line therapy
In the setting of first-line therapy, the major changes in the new guidelines compared with the 2006 ESC guidelines are the downgrading of beta-blockers/upgrading of CCBs, the strong distinction between dihydropyridines and non-dihydropyridine CCBs and the presence of robust statements regarding the combination of CCBs with beta-blockers.
Similarly to the National Institute for Health and Care Excellence (NICE) guideline,23 as the first-line treatment options for angina/ischaemia relief, the 2013 ESC guidelines recommend beta-blockers and/or CCBs to reduce symptoms by decreasing heart rate and contractile response (class I, level of evidence (LoE) A) (Figure 2).1 This differs from the previous ESC guidelines, which recommended starting treatment with a beta-blocker (class I, LoE A) and to attempt monotherapy with a CCB only when beta-blocker therapy is not tolerated or is poorly effective (class I, LoE A).2 A long-acting nitrate or nicorandil were recommended as alternative drugs for monotherapy (class I, LoE C).2 However, the NICE guidelines present some differences compared to the 2013 ESC guidelines; for example, in the case of patients who cannot tolerate beta-blockers or CCBs, or when both are contraindicated, NICE guidelines recommend a monotherapy with a long-acting nitrate, ivabradine, nicorandil or ranolazine; the choice of drug should be based on comorbidities, contraindications, patient preferences and cost. In addition, the NICE guidelines do not mention trimetazidine (it is not available in the UK) among the combination treatment options to be used when symptoms are not successfully controlled with a beta-blocker or a CCB.23
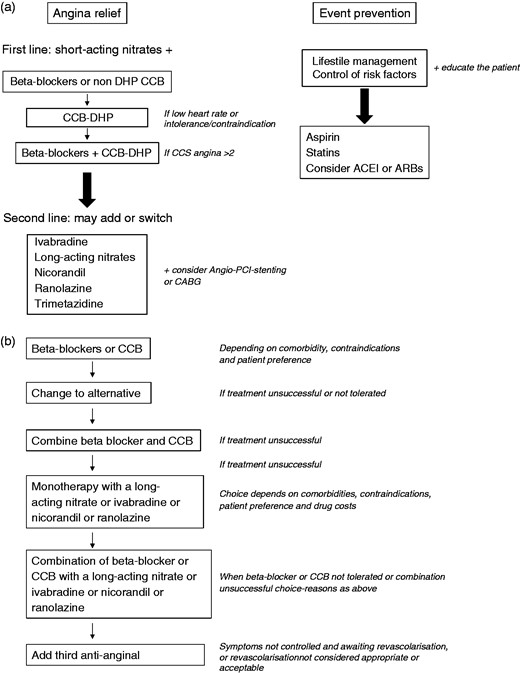
Flow chart of SCAD treatment according to ESC (panel A) and NICE guidelines (panel B).
ACEI: angiotensin-converting enzyme inhibitor; ARBs: angiotensin receptor blockers; CABG: coronary artery bypass grafting; CCBs: calcium channel blockers; CCS: Canadian Cardiovascular Society; DHP: dihydropyridine; PCI: percutaneous coronary intervention.1,23
Although the 2013 guidelines apparently put beta-blockers and CCBs on the same level, it should be noted that they also indicate a possible preference for the former as first-line agents, based on the evidence for prognostic benefits from the use of beta-blockers in post-myocardial infarction patients, or in heart failure.1
However, the upgrading of CCBs to first-line drugs does not appear to be based on any new evidence, because no new large trials on CCBs in SCAD patients have been published since the 2006 guidelines were issued. On the other hand, there has never been any clear evidence of the superiority of beta-blockers to CCBs in the treatment of angina; beta-blockers have been used as the gold standard, at least in part because of their efficacy in multiple heart diseases such as ischaemia, myocardial infarction, arrhythmias. Of note, although the use of beta-blockers and CCBs in SCAD is supported by some evidence24 and clinical experience, most studies were conducted 20 years ago or even before, that is, prior to the implementation of more effective pharmacological strategies for cardiovascular prevention and when the methodology for conducting clinical trials was relatively less rigorous as compared with now. Furthermore, recent indirect evidence from registries and analysis of the use of anti-ischaemic drugs in secondary prevention trials suggest that beta-blockers are not associated with improvement in clinical outcomes in SCAD patients25 and the only large clinical trial with a CCB, including over 6000 patients, failed to demonstrate any benefit for amlodipine.26
Heart rate control is emphasised as a treatment goal for medical therapy in the recent ESC guidelines. In this light, beta-blockers or non-dihydropyridine CCBs should be used as first-line anti-anginal drugs.1 Of course, beta-blockers in particular have more widespread contributory haemodynamic effects in addition to heart rate limitation. Nevertheless, the guidelines do not clearly indicate whether non-dihydropyridine CCBs should be preferred over dihydropyridine CCBs as first-line monotherapy agents,1 although the latter do not reduce heart rate. Furthermore, although the guidelines emphasise the importance of heart rate lowering and despite the evidence that this strategy may be beneficial,27,28 it appears that there is not enough evidence to establish definitively whether a heart rate-lowering strategy is better than a strategy that does not impact heart rate.
After publication of the ESC guidelines, the randomised, double-blind, placebo controlled SIGNIFY trial evaluated ivabradine (up to 10 mg twice daily, with the dose adjusted to achieve a target heart rate of 55–60 beats per minute (bpm) added to standard background therapy in 19,102 patients with SCAD without clinical heart failure and a heart rate of 70 bpm or greater.29 The primary endpoint was a composite of death from cardiovascular causes or non-fatal myocardial infarction. At 3 months, the heart rate of the patients averaged 60.7 ± 9.0 bpm in the ivabradine group compared with 70.6 ± 10.1 bpm with placebo. After a median follow-up of 27.8 months, no significant difference between the ivabradine group and the placebo group in the incidence of the primary endpoint was seen (6.8% and 6.4%, respectively; hazard ratio 1.08; 95% confidence interval 0.96 to 1.20; P = 0.20). However, in a prespecified subgroup of patients with symptomatic angina (Canadian Cardiovascular Society class II–IV) without heart failure, ivabradine showed a small but significant increase in the combined risk of cardiovascular death or non-fatal myocardial infarction.29 According to these findings, it has been suggested that clinicians should ‘exercise caution’ in using ivabradine in moderate to severe angina patients,30 for example, by limiting its prescription to patients with a baseline heart rate of 70 bpm or greater and limiting induced bradycardia to no less than 50 bpm.31,32
With respect to combination therapy, current ESC guidelines state that combination therapy of beta-blockers with non-dihydropyridine CCBs should be avoided because of the risk of bradycardia or atrioventricular block. Conversely, the combination of beta-blockers and dihydropyridine CCBs, such as amlodipine, has been shown to be more effective than single monotherapies, although according to limited evidence,1 and is now considered a first-line treatment option, although often underused in clinical practice.
In patients with microvascular angina, symptomatic treatment remains empirical because of the limited knowledge of the causes that underlie this condition. This is compounded by the lack of suitable techniques to investigate the occurrence of ischaemia at the microvascular level in clinical practice. In particular, while dysfunction of coronary microcirculation can be investigated by various approaches (including non-invasive assessment of coronary flow reserve by transthoracic echocardiography, or positron-emitting tomography, or magnetic resonance imaging), there is no easy and/or accurate means to quantify the ‘extent’ of microcirculation ischaemia. Nuclear cardiology techniques rely on measurements of relative differences of tracer uptake in different areas of the left ventricle. Thus, while they can easily detect regional ischaemia (as in the case of coronary artery stenosis), they are ill-suited to visualize a rather uniform decrease of tissue perfusion across the left ventricle, as is the case with microvascular dysfunction. As for positron-emitting tomography, its use is confined to relatively few institutions, and no trials have investigated its use to guide therapy in patients with microvascular ischaemia.33,34
Thus, at present, beta-blockers may constitute the first choice of therapy for the reduction of the number of anginal attacks in patients with microvascular angina, while CCBs and long-acting nitrates are more helpful when used in addition to beta-blockers in the case of inadequate symptom control. Of note, the ESC guidelines state that new anti-ischaemic drugs such as ranolazine or ivabradine have shown good effects in some patients with microvascular angina.1 In a 4-week randomised study on 46 patients with stable microvascular angina not controlled by standard anti-ischaemic therapy, both ivabradine (5 mg twice a day) and ranolazine (375 mg twice a day) improved the Seattle angina questionnaire (SAQ), the EuroQoL scale and the exercise stress test compared with placebo (P < 0.01 for all), with ranolazine showing some more significant effects compared with ivabradine on some SAQ items and the EuroQoL scale (P < 0.05).35 In addition, time to 1 mm ST-segment depression and exercise duration were improved by ranolazine compared with placebo. Additional but preliminary evidence in favour of ranolazine to treat microvascular angina comes from small studies or post-hoc analysis, which have shown anti-anginal effects in patients (mostly women) with angiographically normal coronary arteries and documented impairment of coronary flow reserve.36,37
Despite these results, there is a clear need for more research in the field of microvascular angina because the available evidence is limited.
Second-line therapy
With regard to second-line treatment, the ESC guidelines recommend adding to first-line drugs any of several drugs, taking into account heart rate, blood pressure and tolerance (class IIa, LoE B) (Figure 2). However, the guidelines make no clear distinction among various second-line drugs, despite the evidence in favour of each agent being of different strength. Indeed, long-acting nitrates, nicorandil, ivabradine and ranolazine, have all been given class IIa, LoE B indication. However, while novel agents such as ranolazine and ivabradine have been put under rigorous testing, and data from large randomised controlled trials are available that support their efficacy as anti-anginal therapies,27–29,38–48 data supporting the use of other agents are more heterogeneous, and the evidence is derived from older and often smaller trials.49–52 In some cases, the guidelines themselves state that prolonged therapy with some of these agents, for example, isosorbide dinitrate, is not even evidence based.1 Furthermore, the efficacy of long-acting nitrates may be limited by the development of tolerance, and worsening of endothelial function is a potential complication associated with the use of these agents.1,23 Nicorandil, a nitrate derivative of nicotinamide having both nitrate-like and ATP-sensitive potassium-channel (K+ATP) activating properties, has been associated with a significant 17% reduction in cardiovascular events in a single trial in 5126 patients with SCAD.53 However, it should be noted that the availability of this agent across Europe is very limited, and at present there is no relevant evidence for nicorandil with regard to clinical and exercise tolerance outcomes.
The use of trimetazidine, an anti-ischaemic metabolic modulator is identified as a second-line option by the ESC guidelines, but is only supported by small trials conducted many years ago, and it is given a class IIb LoE B recommendation. This agent was previously indicated to prevent angina symptoms, and also as an ancillary treatment for vertigo, tinnitus and visual disturbances due to vascular causes.54 However, after a benefit/risk ratio reassessment by the European Medicines Agency (EMA), the therapeutic indication of trimetazidine has been restricted, and it is now indicated only as add-on therapy for the symptomatic treatment of patients with stable angina pectoris who are inadequately controlled by or intolerant to first-line anti-anginal therapies.55 Furthermore, the EMA recommended new contraindications and warnings to reduce and manage the possible risk of tremor, muscle rigidity, restless leg syndrome and Parkinsonism associated with the use of trimetazidine.54
SCAD patients with DM usually have more extensive vessel disease than patients with coronary artery disease alone, are at higher risk of adverse cardiovascular events and have a greater angina burden.1 The ESC guidelines mention that trimetazidine improved glycaemic control in patients with DM, as assessed by glycated haemoglobin (HbA1c) and glycaemia during a small study including 16 male patients with diabetes and ischaemic cardiomyopathy.1,55 However, no information is provided on the anti-anginal efficacy of trimetazidine in this particular population.
On the other hand, the double-blind, placebo controlled Combination Assessment of Ranolazine in Stable Angina (CARISA) trial evaluated 823 SCAD patients on standard treatment who were randomly assigned to placebo or ranolazine (750 mg or 1000 mg) twice daily for 12 weeks.38 Both doses of ranolazine increased exercise duration, prolonged time to angina and to ECG ischaemia, and reduced the weekly number of angina attacks and nitroglycerin consumption. A post-hoc analysis of this trial evaluated the effects of ranolazine in patients with (n = 189) and without (n = 634) DM.56 Ranolazine improved exercise duration, time to onset of angina and to 1 mm ST segment depression both in DM and non-DM patients.56 The same study investigated the effects of ranolazine on glucose homeostasis, assessed by changes in HbA1c, in DM patients with SCAD (n = 131). As compared with placebo, ranolazine 750 and 1000 mg twice daily reduced HbA1c from baseline (by 0.48%, P < 0.008; and 0.7%, P = 0.0002, respectively). Furthermore, more patients on ranolazine 1000 mg had an HbA1c less than 7%, that is, the recommended target HbA1c.57,58
In the 6560 patients with recent non-ST elevation ACSs enrolled in the MERLIN-TIMI 36 (Metabolic Efficiency with Ranolazine for Less Ischaemia in Non-ST-Elevation Acute Coronary Syndromes: Thrombolysis In Myocardial Infarction 36) trial ranolazine reduced recurrent ischaemia and the need for dose increase or the addition of anti-anginal therapy, but had no effect on the primary composite endpoint of cardiovascular death, myocardial infarction or recurrent ischaemia.59 However, in the subgroup of patients with prior chronic angina (n = 3565, 54%), the primary endpoint was less frequent with ranolazine (P = 0.017).60 Diabetes patients on ranolazine were more likely to achieve a HbA1c of less than 7% at 4 and 8 months. In addition, in patients with DM ranolazine reduced the incidence of an increase in HbA1c by 1% or greater compared with placebo. Notably, in patients without DM at baseline, the incidence of new fasting glucose greater than 110 mg/dL or HbA1c of 6% or greater was reduced by ranolazine (31.8% vs. 41.2%; P = 0.003). In another analysis of data from the MERLIN-TIMI 36 trial,61 the placebo-corrected reduction in HbA1c with ranolazine was 0.28% (P = 0.045) in diabetes patients with better glycaemic control (HbA1c ≥ 6% to < 8%) and 0.59% (P < 0.001) in subjects with poorer glycaemic control (HbA1c ≥ 8% to 10%). Moreover, in patients with severe hyperglycaemia at baseline (fasting plasma glucose (FPG) ≥ 150–400 mg/dL) ranolazine decreased FPG, whereas there was no change in patients with baseline FPG less than 150 mg/dL.62 Furthermore, in the prospective TERISA (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina) trial in DM patients with coronary artery disease (n = 949), ranolazine significantly reduced the weekly angina frequency (3.8 vs. 4.3 episodes; P = 0.008) and sublingual nitroglycerin use (1.7 vs. 2.1 doses) compared with placebo.39
Taken together, as also evidenced by the ESC guidelines, these results suggest that ranolazine can be added to other well established anti-anginal drugs, particularly in patients with higher HbA1c levels.1,63
Finally, the guidelines mention allopurinol, an inhibitor of xanthine-oxidase used for lowering uric acid in people with gout, as an agent with anti-anginal properties. Although it has been suggested that reducing oxidative stress via inhibition of xanthine-oxidase might be beneficial in patients with CVD,64,65 the evidence supporting the role of allopurinol for the treatment of SCAD is limited to two randomised, crossover studies in which a high dose od allopurinol was used,65,66 and therefore the potential benefits of this agent should be weighed against the associated risk. However, it is interesting to note that current SCAD guidelines consider xanthine-oxidase and uric acid as important targets for the control of cardiovascular risk, in line with available evidence.67
Overall, the ESC guidelines provide little precise guidance to physicians as to the choice of a second-line agent. However, the implication of the guidelines is that drugs that lower blood pressure, such as long-acting nitrates should be avoided in patients with hypotension, that drugs such as ivabradine should not be used in patients with bradycardia and that ranolazine might be considered in patients with bradycardia or hypotension. In addition, some indications may even be confusing. Both ranolazine and trimetazidine are indicated as drugs with data in diabetes patients.1 However, based on the evidence reviewed in the same guidelines,41,55 it appears that ranolazine may be a more appropriate option in this patient population. Furthermore, no recommendations are given with respect to OMT in important ‘special’ patient populations. These include, but are not limited to, women, patients with kidney disease, the elderly and those affected with hypotension. Moreover, the guidelines do not provide specific recommendations for patients with chronic obstructive pulmonary disease in whom non-selective beta-blockers should be used with caution.68–70
Similarly, there is no advice for the treatment of patients with persistent angina after revascularisation. In particular, with regard to the latter population, it remains unclear whether anti-anginal treatment should be continued after revascularisation or rather it should be stopped and instituted again only if the patient remains symptomatic. OMT should be individualised according to patient characteristics (e.g. heart rate, severity of symptoms, presence of comorbidities), and the choice of an add-on therapy should also be supported by clear evidence in favour of the efficacy of the drug. Interestingly, the results of a recent meta-analysis indicate that the combination of a beta-blocker and a CCB with each other or one of them with ranolazine show a significant improvement in exercise parameters, angina frequency and nitrate use, whereas ivabradine demonstrated some benefits for the exercise tolerance test, but these were not matched in clinical domains, and no data are available for nicorandil (Figure 3).52
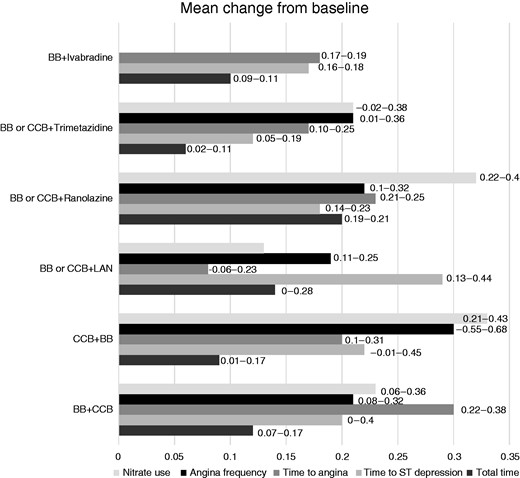
Results of a meta-analysis of available evidence on add-on treatment in SCAD patients. The mean change from baseline of outcomes is represented. Numbers next to bars are confidence intervals. BB: β-blocker; CCBs: calcium channel blockers; LAN: long-acting nitrate.52
Revascularisation
The guidelines provide recommendations for myocardial revascularisation, which were recently updated in a dedicated ESC guidelines (reference ESC guidelines on revascularisation).71 Revascularisation is highly recommended in patients with ongoing angina in spite of OMT, as well as in high-risk populations. However, the possible benefit of myocardial revascularisation has not been established in stable patients without a coronary anatomy suggestive of very high risk for outcomes.
The currently ongoing ISCHEMIA trial (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches; NCT01471522), a multicentre, randomised controlled trial with an enrolment target of 8000 patients, will help determine the best management strategy for high-risk patients with SIHD by comparing the efficacy of an initial invasive strategy (cardiac catheterisation plus revascularisation and OMT) with that of a conservative strategy (OMT alone with catheterisation reserved for failure of OMT) on the primary composite endpoint of time to first occurrence of cardiovascular death or non-fatal myocardial infarction. In addition, the ISCHEMIA trial might shed light on other issues that remain to be clarified. In fact, the definition of OMT also impacts the subsequent management of SCAD patients, because the decision regarding proceeding to angiography and revascularisation is based on the response to medical therapy.
Conclusions
The 2013 guidelines on the management of SCAD have brought some novelty to the management of this disease. OMT as well as myocardial revascularisation for SCAD should be specified and supported by robust evidence. The ESC guidelines have identified but left behind some ‘grey areas’ which require clarification and further future investigation.
Acknowledgements
Editorial assistance has been provided by Content Ed Net. This activity was sustained by an unconditional grant from Menarini International. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AJC is an advisor to Menarini and Gilead and Servier; JT serves on a speaker's bureau for Menarini; GA is on the advisory boards of Merck, Angelini, Menarini; and speaker's bureaux of Merck, Boheringer, Menarini; AM received research grants from Menarini International, Gilead and Servier.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.




Comments