-
PDF
- Split View
-
Views
-
Cite
Cite
Jiao Luo, Ida Juul Rasmussen, Børge G Nordestgaard, Anne Tybjærg-Hansen, Jesper Qvist Thomassen, Ruth Frikke-Schmidt, Cardiovascular diseases and risk of dementia in the general population, European Journal of Preventive Cardiology, 2025;, zwaf129, https://doi.org/10.1093/eurjpc/zwaf129
Close - Share Icon Share
Abstract
Cardiovascular diseases (CVDs) have been linked to increased risk of dementia in observational studies, whereas genetic studies have yielded inconsistent findings. We aimed to determine whether nine CVDs are causally associated with the risk of all-cause dementia, Alzheimer’s disease, and vascular dementia.
We performed time-dependent Cox regression analyses in three prospective cohorts, the Copenhagen City Heart Study (n = 10 373), the Copenhagen General Population Study (n = 101 582), and the UK Biobank (n = 377 706) and meta-analysed individual estimates. Furthermore, we assessed genetic susceptibility for CVDs and the risk of dementia using individual-level data from the UK Biobank and summary statistics from the FinnGen study. Observationally, CVDs were associated with risk of all incident outcomes in meta-analyses, with hazard ratios up to 7.00 (95% confidence interval: 6.20, 7.92). Genetically, in the UK Biobank, susceptibility for ischaemic stroke was associated with risk of all-cause dementia, Alzheimer’s disease, and vascular dementia, and odds ratios (ORs) were 1.64 (1.35, 1.98), 1.44 (1.10, 1.89), and 2.06 (1.41, 3.01), respectively, with similar estimates for ischaemic stroke; genetic susceptibility for ischaemic heart disease was associated with risk of vascular dementia [OR: 1.24 (1.03, 1.50)]. Genetic summary statistics from the FinnGen study confirmed the associations between ischaemic stroke and stroke.
Associations between stroke and all-cause dementia and its major subtypes are likely to be causal. Moreover, genetic susceptibility for ischaemic heart disease is associated with incident vascular dementia. These findings underscore the importance of integrating CVD prevention into interventions to enable early prevention and reduce the risk of dementia.
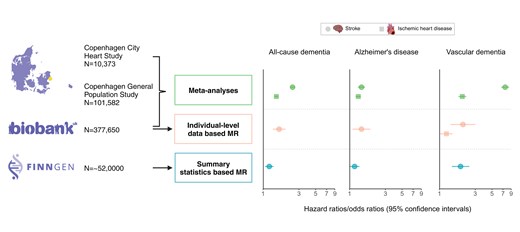
Lay Summary
This study used observational and genetic studies using Mendelian randomization (MR) design to triangulate the causal impact of cardiovascular disease (CVDs) on dementia.
We confirmed the concordant observational association between eight CVDs and dementia using three large cohorts with ∼0.5 million participants.
Through unbiased comprehensive one- and two-sample MR analyses, we substantiate causal associations between stroke and dementia and between ischaemic heart disease and vascular dementia.
Introduction
Cardiovascular diseases (CVDs) remain the leading cause of mortality and disability worldwide, posing substantial health and economic challenges for individuals and societies.1 The updated 2024 report of the Lancet Commission for dementia prevention, intervention, and care has emphasized that CVDs and dementia share many risk factors.2 Many cardiovascular events occur in mid to early late life and thus predispose individuals to a high risk of diseases of late life, particularly dementia. In the absence of efficient and disease-modifying treatments for dementia, identifying causal risk factors that enable early targeted and effective interventions is of paramount importance.
The biological link between CVDs and dementia engages multiple complex mechanisms. In addition to several shared cardiovascular risk factors,3–7 vascular damage and dysfunction derived from CVDs across the cerebral vasculature into the brain may contribute to beta-amyloid burden and may thus increase dementia risk individually and synergistically.8,9 Moreover, genetic factors are directly involved in the onset and development of both CVDs and dementia, and genome-wide association studies (GWAS) have identified shared genetic factors between CVDs and dementia in lipid metabolism and the immune system.10,11 A large number of observational studies have shown that individuals with cardiovascular comorbidities are at higher risk of dementia—a risk that increases stepwise from single to multiple comorbidities, with disease severity and with rapid disease development and progression.12–22 Due to the observational design, these associations are however susceptible to inherited limitations such as residual confounding. Moreover, dementia has a long prodromal phase where the symptoms are not well-manifested despite pathological alterations in the brain. This makes it extremely difficult to determine the sequential order of the studied CVDs and dementia, introducing reverse causation. Hence, the observed associations cannot suggest causality.
Mendelian randomization (MR) largely avoids these limitations by using genetic variants as instrumental variables for exposure and has been widely used to establish causal associations for risk factors and drug targets. Although many MR studies on CVDs and dementia using summary data have been conducted, the results are conflicting.23–28 Several explanations are proposed to account for this controversy, and one important criticism lies in survival bias, especially when performing MR studies in late-life diseases, such as dementia. This is because individuals with a high risk of CVD could have died before getting dementia and have erroneously been classified as having a low dementia risk. Consequently, in previous summary data–based two-sample MR studies, CVDs have been incorrectly shown to confer a lower risk of Alzheimer’s disease.26–28 Therefore, robust evidence for the potential causal nature of the associations between preceding CVDs and subsequent development of all-cause dementia and its major subtypes has not until now been established. Due to a lack of proper statistical solutions, analyses based on a prospective general population cohort can mitigate survival bias and consequently draw robust conclusions in MR analyses of dementia.
Following the World Health Organization’s global burden of disease estimates, we identified nine individual CVDs that contribute most to death and disability, including ischaemic heart disease, myocardial infarction, peripheral arterial disease, hypertensive heart disease, stroke, ischaemic stroke, haemorrhagic stroke, atrial fibrillation, and heart failure. First, we analysed data from three large-scale prospective cohorts separately and then in meta-analyses to systematically examine the associations between these CVDs and incident all-cause dementia, Alzheimer’s disease, and vascular dementia. Second, we performed rigorous and unbiased MR analyses to investigate the associations between genetic predisposition to CVDs and the risk of dementia, using individual-level data from the UK Biobank and summary statistics from the FinnGen study.
Methods
The study design is illustrated in Figure 1. Each included study has been approved by local ethical committees.
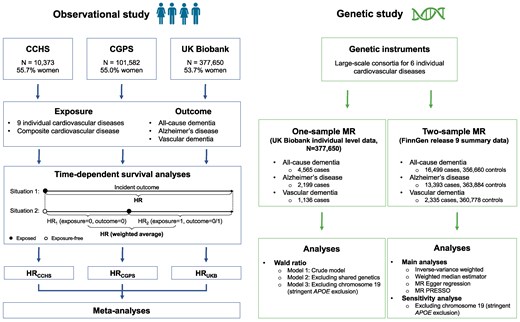
Study design. We performed observational and genetic studies. Observational studies were carried out in three prospective cohorts, respectively, using time-dependent survival analysis, where the follow-up time for each patient is divided into different time windows according to the status of the exposure. For each time window, a separate Cox analysis is carried out using the value of the time-dependent cardiovascular diseases at the beginning of that specific time window. Subsequently, a weighted average of all the time window-specific results is computed. Estimates from individual cohorts were meta-analysed using both random- and fixed-effects models. In genetic analyses, we performed one-sample Mendelian randomization using individual-level data from the UK Biobank and two-sample MR using summary statistics from the FinnGen study. CCHS, Copenhagen City Heart Study; CGPS, Copenhagen General Population Study; HR, hazard ratio; MR, Mendelian randomization. Figure is made with BioRender.
Observational study
Study cohorts
Copenhagen cohorts
We included participants from two similar prospective cohorts of the Danish general population with covariate measurements and free of dementia at baseline, the Copenhagen City Heart Study (CCHS, n = 10 373) and the Copenhagen General Population Study (CGPS, n = 101 585). Both studies were approved by the institutional review boards and Danish ethical committees. Written informed consent was obtained from all participants. The CCHS was initiated in 1976–78, with follow-up examinations in 1981–83, 1991–94, and 2001–03, with a median follow-up time of 15 (<1–27) years. The CGPS was initiated in November 2003 with ongoing enrolment, with a median follow-up time of 9 (<1–14) years. Individuals were randomly selected from the national Danish Civil Registration System to reflect the adult population aged 20 years or older. No individuals were lost to follow-up due to the unique Danish registries. Relevant data were obtained from a self-administered questionnaire, a physical examination, and a blood test. In addition to data collected within the study, disease and death status were available by linkage to the national Danish Patient Registry and the Danish Causes of Death Registry through a unique personal identification number.
UK Biobank
The UK Biobank is a prospective cohort with 502 628 participants between the ages of 40 and 69 years recruited from the general population at multiple assessment centres across the UK between 2006 and 2010. The UK Biobank was approved by the North-West Multi-center Research Ethics Committee. Detailed information about the study design, investigation methods, ethical approval, and limitations has been reported previously.29 Diseases were ascertained by linking to health data for all participants, including deaths, primary care data, and hospital inpatient records, which are gathered from several separate national data providers. We included 377 650 non-related White British participants free of dementia at baseline with available genetic information in the study, with a median follow-up of 13 (<1–16) years.
Disease endpoints
Exposures and outcomes were identified according to the International Classification of Disease (ICD) codes. In the Copenhagen cohorts, diagnoses were based on ICD8 and ICD10 codes, as ICD9 codes never have been implemented in Denmark, and were collected through the national Danish Patient Registry which contains information on all patient contacts with public and private clinical hospital departments from 1977, including emergency wards and outpatient clinics from 1995. Cause of death was obtained from the national Danish Causes of Death Registry, as reported by hospitals and general practitioners since 1977. In the UK Biobank, the ascertainment of diseases comes from the linkage to electronic health records, including inpatient hospital records, primary care records, and death registrations. Diseases were defined using a combination of ICD9 and ICD10 codes together with self-reported illness (coded by the UK Biobank); self-reported diagnosis was only used in CVDs. All relevant codes for each endpoint for all cohorts are available in the Supplementary material online, Table S1. The respective censoring dates were emigration or the last update of the registries of December 2018 for the Copenhagen cohorts and March 2022 for the UK Biobank.
Exposures and outcomes
Exposures and outcomes were identified using the International Classification of Disease codes (see Supplementary material online, Table S1). Following the global burden of disease, we assessed nine CVDs that have largely contributed to the death and disability with available data: ischaemic heart disease, myocardial infarction, peripheral arterial disease, hypertensive heart disease, stroke, ischaemic stroke, haemorrhagic stroke, atrial fibrillation, and heart failure. We also generated an additional combined measure including any examined individual CVD that assessed the overall impact of cardiovascular health on the outcomes. This was documented as the first recorded diagnosis of any examined CVD. We examined three dementia outcomes: all-cause dementia, Alzheimer’s disease, and vascular dementia. All-cause dementia comprises Alzheimer’s disease, unspecified dementia, and vascular dementia. We combined unspecified dementia and vascular dementia into vascular-related dementia due to their shared vascular risk factors (see Supplementary material online, Table S1).
Covariates
Confounding factors were chosen primarily guided by empirical evidence (from previous studies) or theoretical knowledge of suspected or established confounding factors. They were included in the Cox regression model one by one, and we compared the estimated changes of the exposure before and after introducing a certain confounding factor, as well as compared the models with and without this factor using a likelihood ratio test. If a potential confounding factor had a notable influence, defined by a 10% change in the exposure estimates or a significant P-value through model comparison, it was included in the final choice of adjusting covariates. The selection process was performed for each cohort, and in the final analytic model, we included all potential covariates identified in any cohorts.
We generated similar covariates from baseline characteristics for CCHS, CGPS, and the UK Biobank, except for alcohol consumption. This is because the amount of reported total alcohol intake in the UK Biobank has a substantial number of missing values, thus not being suitable for multiple imputation. Covariates included age and sex, body mass index (BMI, kg/m2), educational attainment (schooling years < 8 years and ≥8 years), smoking status (self-reported current, former, and never smoker), alcohol intake levels (high or low in the Copenhagen cohorts; high intake was defined as >14/21 units per week for women/men where 1 unit equals 12 g alcohol, equivalent to one glass of wine or one 33 cL beer; frequency in the UK Biobank as <1 time, one to four times per week and daily or almost daily), physical activity (≥4 or <4 h per week), plasma total cholesterol (mmol/L), lipid-lowering therapy (>97% statins in the Copenhagen cohorts, yes/no), systolic and diastolic blood pressure (mmHg), C-reactive protein (mg/L), antihypertensive medication (yes/no), and apolipoprotein E (APOE) genotype, for which ε4 and ε2 alleles were defined by p.Cys130Arg (rs429358) and p.Arg176Cys (rs7412), respectively, and were grouped into six common genotypes: ε22, ε32, ε42, ε33, ε43, and ε44. Moreover, several important chronic conditions were also included, including Type 2 diabetes (yes/no), any type of cancer (yes/no), and estimated glomerular filtration rate (eGFR).30
Statistical analyses
All analyses were undertaken using R (v4.0.2) statistical software. Baseline characteristics are presented as numbers (frequencies) for categorical variables and medians (interquartile ranges) for continuous variables. Participants who had dementia before the occurrence of CVDs were excluded from the analyses of the specific exposure. We imputed missing values for covariates using multiple imputation by chained equations with 10 imputed datasets. We examined the associations between CVDs and incident dementia using cause-specific Cox regression models, accounting for competing risk of death. Follow-up ended at dementia diagnosis, death, or censoring, whichever came first. We performed regressions using CVDs as a time-varying covariate whenever the exposure occurred after the study baseline. If the exposure was ascertained before the study entry, the follow-up time started from baseline. Four observational models were fitted, Model 1 was adjusted for sex and age as time scale to avoid left truncation; Model 2 was additionally adjusted for education, BMI, smoking, alcohol consumption, physical activity, blood pressure, total cholesterol, antihypertensive and lipid-lowering medication, and C-reactive protein; Model 3 was additionally adjusted for Type 2 diabetes, cancer, and eGFR; and Model 4 was additionally adjusted for APOE genotype. We obtained estimates using Rubin’s rules to combine the results from each imputed dataset. All the analyses were performed for each cohort separately and then combined using random-effect models in meta-analyses and between-cohort heterogeneity was quantified using I2. However, when the number of studies in a meta-analysis is small, the estimation of the heterogeneity is imprecise and unreliable, thus leading to potentially biased or unstable estimates of the overall effect size.31,32 Fixed-effects models are simpler and avoid the additional uncertainty. Therefore, we also used fixed-effects models to enhance the robustness of our findings. Results were presented as hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). As the exposures and outcomes were inter-correlated, we additionally adjusted P-values using a false discovery rate (significance level at 0.05) per model to account for multiple tests.
In sensitivity analyses, we (i) stratified by sex; (ii) stratified by APOE genotype (carriers of no vs. one or more ε2 or ε4 alleles); (iii) repeated the analyses for vascular-related dementia; (iv) repeated the analyses in participants free of incident dementia up to 12 months after the index date of CVDs for the analyses of specific CVDs, to minimize the risk of reverse causation and maximally ensure statistical power; and (v) examined the associations between CVDs and mild cognitive impairment (ICD10: F067). This has only been performed in the UK Biobank due to the limited cases, and thus insufficient power, in the Copenhagen cohorts. Additionally, we also investigated the association between cardiovascular multimorbidity (≥2 and ≥3 vs. ≤1 of ischaemic heart disease, peripheral arterial disease, hypertensive heart disease, stroke, atrial fibrillation, and heart failure) and risk of dementia.
Genetic study
We selected independent single-nucleotide polymorphisms (SNPs) at a genome-wide significance level (P < 5 × 10−8) from the largest CVD genomic consortia presented in the original publication (see Supplementary material online, Table S2). Odds ratios (ORs) represent the risk of dementia per logOR increase of CVDs. We reported original P-values in the main results, and multiple testing was corrected for per MR method using a false discovery rate.
One-sample Mendelian randomization analyses
We used individual-level data from the UK Biobank described in the observational study earlier. For each CVD, we calculated a genetic risk score (GRS) for all participants as a weighted sum of the effect size estimates and the genotype of each selected variant:
Two-sample Mendelian randomization analyses
The MR analyses using data from GWAS with case-control study design from consortia are vulnerable to survival bias in dementia,33,34 as individuals should have survived long enough to be enrolled. The GWAS using nested case-control design within a prospective cohort may mitigate this issue as individuals were followed from a relatively young age and were possibly largely free from CVDs at baseline. Therefore, we used summary statistics for all-cause dementia, Alzheimer’s disease, and vascular dementia from FinnGen using the same ICDs as for other cohorts. The FinnGen study is an ongoing cohort study launched in 2017, which included the genetic data generated from biobank samples and health-related data from social and healthcare registers. The FinnGen study was approved by the Coordinating Ethics Committee of the Hospital District of Helsinki, and GWAS were performed across a broad spectrum of phenotypes, adjusted for age, sex, principal components, and genotype batch effect. Detailed information regarding participants for GWAS, genotype platforms, and statistical analysis protocols is available at the FinnGen website (https://www.finngen.fi/en). We used the 9th data release (May 2023), including 16 499 all-cause dementia cases and 356 660 controls, 13 393 Alzheimer’s disease cases and 363 884 controls, and 2335 vascular dementia cases and 360 778 controls. The median age (63 years35) of participants from FinnGen was significantly younger than that from the largest Alzheimer’s disease consortium.10 The SNPs directly associated with any outcome were excluded. We performed analyses including the inverse-variance weighted (IVW), weighted median estimator, MR-Egger, and MR-PRESSO that allows for evaluation and correction for horizontal pleiotropy via outlier removal.
Results
Observational study
Baseline characteristics for CCHS, CGPS, and the UK Biobank are shown in Table 1. The median ages were 45.6 (36.7, 54.5), 57.7 (48.0, 67.1), and 59.0 (51.0, 64.0) years, and the percentages of women were 55.7%, 55.0%, and 53.7%, respectively.
| . | CCHS . | CGPS . | UKB . |
|---|---|---|---|
| n | 10 373 | 101 582 | 377 650 |
| Age, years | 45.6 (36.7, 54.5) | 57.7 (48.0, 67.1) | 59.0 (51.0, 64.0) |
| Sex | |||
| Men | 4598 (44.3) | 45 691 (45.0) | 174 823 (46.3) |
| Women | 5775 (55.7) | 55 891 (55.0) | 202 827 (53.7) |
| Educational attainment, years | |||
| <8 | 3219 (31.0) | 9979 (9.8) | 68 924 (18.3) |
| ≥8 | 7154 (69.0) | 91 607 (91.2) | 308 726 (81.7) |
| APOE genotype | |||
| e33 | 5823 (56.1) | 56 471 (55.6) | 219 660 (58.2) |
| e43 | 2619 (25.2) | 25 873 (25.5) | 90 028 (23.8) |
| e32 | 1305 (12.6) | 12 644 (12.4) | 46 749 (12.4) |
| e42 | 283 (2.7) | 2940 (2.9) | 9678 (2.6) |
| e44 | 284 (2.7) | 2943 (2.9) | 9103 (2.4) |
| e22 | 59 (0.6) | 711 (0.7) | 2432 (0.6) |
| Smoking status | |||
| Never | 2682 (25.9) | 43 164 (42.5) | 205 645 (54.5) |
| Current | 5821 (56.1) | 17 599 (17.3) | 134 269 (35.6) |
| Former | 1870 (18.0) | 40 819 (40.2) | 37 736 (10.0) |
| Physical activity, hours per week | |||
| ≥4 | 3683 (35.5) | 52 356 (51.5) | 290 511 (76.9) |
| <4 | 6690 (64.5) | 49 226 (48.5) | 87 139 (23.1) |
| Alcohol consumptiona | |||
| Low | 5887 (56.8) | 89 633 (88.2) | — |
| High | 4486 (43.2) | 11 949 (11.8) | — |
| Alcohol intake frequencyb | |||
| <1 time per week | — | — | 106 592 (28.2) |
| 1–4 times per week | — | — | 190 066 (50.3) |
| Daily or almost daily | — | — | 80 992 (21.4) |
| Body mass index, kg/m2 | 23.9 (21.8, 26.6) | 25.6 (23.2, 28.4) | 26.8 (24.2, 29.9) |
| Total cholesterol, mmol/L | 5.7 (4.9, 6.5) | 6.5 (5.6, 7.3) | 5.7 (4.9, 6.5) |
| Lipid-lowering therapy | |||
| Yes | 18 (0.2) | 11 979 (11.8) | 67 954 (18.0) |
| No | 10 355 (99.8) | 10 002 (96.4) | 309 696 (82.0) |
| Systolic blood pressure, mmHg | 127.0 (116.0, 140.0) | 140.0 (126.0, 155.0) | 137.0 (125.5, 150.5) |
| Diastolic blood pressure, mmHg | 80.0 (73.0, 88.0) | 84.0 (76.0, 91.0) | 82.0 (75.5, 89.0) |
| Antihypertensive medication | |||
| Yes | 371 (3.6) | 20 093 (19.8) | 81 166 (21.5) |
| No | 10 002 (96.4) | 81 489 (80.2) | 296 484 (78.5) |
| Dementia prevalence, n (%) | |||
| All-cause dementia | 1125 (10.8) | 2443 (2.4) | 4565 (1.2) |
| Alzheimer’s dementia | 472 (4.6) | 1588 (1.6) | 2199 (0.6) |
| Vascular dementia | 137 (1.3) | 252 (0.2) | 1136 (0.3) |
| Vascular-related dementia | 908 (8.8) | 1289 (1.3) | 3279 (0.9) |
| . | CCHS . | CGPS . | UKB . |
|---|---|---|---|
| n | 10 373 | 101 582 | 377 650 |
| Age, years | 45.6 (36.7, 54.5) | 57.7 (48.0, 67.1) | 59.0 (51.0, 64.0) |
| Sex | |||
| Men | 4598 (44.3) | 45 691 (45.0) | 174 823 (46.3) |
| Women | 5775 (55.7) | 55 891 (55.0) | 202 827 (53.7) |
| Educational attainment, years | |||
| <8 | 3219 (31.0) | 9979 (9.8) | 68 924 (18.3) |
| ≥8 | 7154 (69.0) | 91 607 (91.2) | 308 726 (81.7) |
| APOE genotype | |||
| e33 | 5823 (56.1) | 56 471 (55.6) | 219 660 (58.2) |
| e43 | 2619 (25.2) | 25 873 (25.5) | 90 028 (23.8) |
| e32 | 1305 (12.6) | 12 644 (12.4) | 46 749 (12.4) |
| e42 | 283 (2.7) | 2940 (2.9) | 9678 (2.6) |
| e44 | 284 (2.7) | 2943 (2.9) | 9103 (2.4) |
| e22 | 59 (0.6) | 711 (0.7) | 2432 (0.6) |
| Smoking status | |||
| Never | 2682 (25.9) | 43 164 (42.5) | 205 645 (54.5) |
| Current | 5821 (56.1) | 17 599 (17.3) | 134 269 (35.6) |
| Former | 1870 (18.0) | 40 819 (40.2) | 37 736 (10.0) |
| Physical activity, hours per week | |||
| ≥4 | 3683 (35.5) | 52 356 (51.5) | 290 511 (76.9) |
| <4 | 6690 (64.5) | 49 226 (48.5) | 87 139 (23.1) |
| Alcohol consumptiona | |||
| Low | 5887 (56.8) | 89 633 (88.2) | — |
| High | 4486 (43.2) | 11 949 (11.8) | — |
| Alcohol intake frequencyb | |||
| <1 time per week | — | — | 106 592 (28.2) |
| 1–4 times per week | — | — | 190 066 (50.3) |
| Daily or almost daily | — | — | 80 992 (21.4) |
| Body mass index, kg/m2 | 23.9 (21.8, 26.6) | 25.6 (23.2, 28.4) | 26.8 (24.2, 29.9) |
| Total cholesterol, mmol/L | 5.7 (4.9, 6.5) | 6.5 (5.6, 7.3) | 5.7 (4.9, 6.5) |
| Lipid-lowering therapy | |||
| Yes | 18 (0.2) | 11 979 (11.8) | 67 954 (18.0) |
| No | 10 355 (99.8) | 10 002 (96.4) | 309 696 (82.0) |
| Systolic blood pressure, mmHg | 127.0 (116.0, 140.0) | 140.0 (126.0, 155.0) | 137.0 (125.5, 150.5) |
| Diastolic blood pressure, mmHg | 80.0 (73.0, 88.0) | 84.0 (76.0, 91.0) | 82.0 (75.5, 89.0) |
| Antihypertensive medication | |||
| Yes | 371 (3.6) | 20 093 (19.8) | 81 166 (21.5) |
| No | 10 002 (96.4) | 81 489 (80.2) | 296 484 (78.5) |
| Dementia prevalence, n (%) | |||
| All-cause dementia | 1125 (10.8) | 2443 (2.4) | 4565 (1.2) |
| Alzheimer’s dementia | 472 (4.6) | 1588 (1.6) | 2199 (0.6) |
| Vascular dementia | 137 (1.3) | 252 (0.2) | 1136 (0.3) |
| Vascular-related dementia | 908 (8.8) | 1289 (1.3) | 3279 (0.9) |
Values are reported as median (interquartile range) for continuous variables and number (percentage) for categorical variables.
CCHS, Copenhagen City Heart Study; CGPS, Copenhagen General Population Study.
aHigh alcohol intake was defined as >14/21 units per week for women/men (1 unit = 12 g alcohol, equivalent to one glass of wine or one beer (33 cL).
bSince the amount of alcohol intake in the UK Biobank has a substantial number of missing values and since this variable is not suitable for multiple imputation, we show the frequency of alcohol intake instead.
| . | CCHS . | CGPS . | UKB . |
|---|---|---|---|
| n | 10 373 | 101 582 | 377 650 |
| Age, years | 45.6 (36.7, 54.5) | 57.7 (48.0, 67.1) | 59.0 (51.0, 64.0) |
| Sex | |||
| Men | 4598 (44.3) | 45 691 (45.0) | 174 823 (46.3) |
| Women | 5775 (55.7) | 55 891 (55.0) | 202 827 (53.7) |
| Educational attainment, years | |||
| <8 | 3219 (31.0) | 9979 (9.8) | 68 924 (18.3) |
| ≥8 | 7154 (69.0) | 91 607 (91.2) | 308 726 (81.7) |
| APOE genotype | |||
| e33 | 5823 (56.1) | 56 471 (55.6) | 219 660 (58.2) |
| e43 | 2619 (25.2) | 25 873 (25.5) | 90 028 (23.8) |
| e32 | 1305 (12.6) | 12 644 (12.4) | 46 749 (12.4) |
| e42 | 283 (2.7) | 2940 (2.9) | 9678 (2.6) |
| e44 | 284 (2.7) | 2943 (2.9) | 9103 (2.4) |
| e22 | 59 (0.6) | 711 (0.7) | 2432 (0.6) |
| Smoking status | |||
| Never | 2682 (25.9) | 43 164 (42.5) | 205 645 (54.5) |
| Current | 5821 (56.1) | 17 599 (17.3) | 134 269 (35.6) |
| Former | 1870 (18.0) | 40 819 (40.2) | 37 736 (10.0) |
| Physical activity, hours per week | |||
| ≥4 | 3683 (35.5) | 52 356 (51.5) | 290 511 (76.9) |
| <4 | 6690 (64.5) | 49 226 (48.5) | 87 139 (23.1) |
| Alcohol consumptiona | |||
| Low | 5887 (56.8) | 89 633 (88.2) | — |
| High | 4486 (43.2) | 11 949 (11.8) | — |
| Alcohol intake frequencyb | |||
| <1 time per week | — | — | 106 592 (28.2) |
| 1–4 times per week | — | — | 190 066 (50.3) |
| Daily or almost daily | — | — | 80 992 (21.4) |
| Body mass index, kg/m2 | 23.9 (21.8, 26.6) | 25.6 (23.2, 28.4) | 26.8 (24.2, 29.9) |
| Total cholesterol, mmol/L | 5.7 (4.9, 6.5) | 6.5 (5.6, 7.3) | 5.7 (4.9, 6.5) |
| Lipid-lowering therapy | |||
| Yes | 18 (0.2) | 11 979 (11.8) | 67 954 (18.0) |
| No | 10 355 (99.8) | 10 002 (96.4) | 309 696 (82.0) |
| Systolic blood pressure, mmHg | 127.0 (116.0, 140.0) | 140.0 (126.0, 155.0) | 137.0 (125.5, 150.5) |
| Diastolic blood pressure, mmHg | 80.0 (73.0, 88.0) | 84.0 (76.0, 91.0) | 82.0 (75.5, 89.0) |
| Antihypertensive medication | |||
| Yes | 371 (3.6) | 20 093 (19.8) | 81 166 (21.5) |
| No | 10 002 (96.4) | 81 489 (80.2) | 296 484 (78.5) |
| Dementia prevalence, n (%) | |||
| All-cause dementia | 1125 (10.8) | 2443 (2.4) | 4565 (1.2) |
| Alzheimer’s dementia | 472 (4.6) | 1588 (1.6) | 2199 (0.6) |
| Vascular dementia | 137 (1.3) | 252 (0.2) | 1136 (0.3) |
| Vascular-related dementia | 908 (8.8) | 1289 (1.3) | 3279 (0.9) |
| . | CCHS . | CGPS . | UKB . |
|---|---|---|---|
| n | 10 373 | 101 582 | 377 650 |
| Age, years | 45.6 (36.7, 54.5) | 57.7 (48.0, 67.1) | 59.0 (51.0, 64.0) |
| Sex | |||
| Men | 4598 (44.3) | 45 691 (45.0) | 174 823 (46.3) |
| Women | 5775 (55.7) | 55 891 (55.0) | 202 827 (53.7) |
| Educational attainment, years | |||
| <8 | 3219 (31.0) | 9979 (9.8) | 68 924 (18.3) |
| ≥8 | 7154 (69.0) | 91 607 (91.2) | 308 726 (81.7) |
| APOE genotype | |||
| e33 | 5823 (56.1) | 56 471 (55.6) | 219 660 (58.2) |
| e43 | 2619 (25.2) | 25 873 (25.5) | 90 028 (23.8) |
| e32 | 1305 (12.6) | 12 644 (12.4) | 46 749 (12.4) |
| e42 | 283 (2.7) | 2940 (2.9) | 9678 (2.6) |
| e44 | 284 (2.7) | 2943 (2.9) | 9103 (2.4) |
| e22 | 59 (0.6) | 711 (0.7) | 2432 (0.6) |
| Smoking status | |||
| Never | 2682 (25.9) | 43 164 (42.5) | 205 645 (54.5) |
| Current | 5821 (56.1) | 17 599 (17.3) | 134 269 (35.6) |
| Former | 1870 (18.0) | 40 819 (40.2) | 37 736 (10.0) |
| Physical activity, hours per week | |||
| ≥4 | 3683 (35.5) | 52 356 (51.5) | 290 511 (76.9) |
| <4 | 6690 (64.5) | 49 226 (48.5) | 87 139 (23.1) |
| Alcohol consumptiona | |||
| Low | 5887 (56.8) | 89 633 (88.2) | — |
| High | 4486 (43.2) | 11 949 (11.8) | — |
| Alcohol intake frequencyb | |||
| <1 time per week | — | — | 106 592 (28.2) |
| 1–4 times per week | — | — | 190 066 (50.3) |
| Daily or almost daily | — | — | 80 992 (21.4) |
| Body mass index, kg/m2 | 23.9 (21.8, 26.6) | 25.6 (23.2, 28.4) | 26.8 (24.2, 29.9) |
| Total cholesterol, mmol/L | 5.7 (4.9, 6.5) | 6.5 (5.6, 7.3) | 5.7 (4.9, 6.5) |
| Lipid-lowering therapy | |||
| Yes | 18 (0.2) | 11 979 (11.8) | 67 954 (18.0) |
| No | 10 355 (99.8) | 10 002 (96.4) | 309 696 (82.0) |
| Systolic blood pressure, mmHg | 127.0 (116.0, 140.0) | 140.0 (126.0, 155.0) | 137.0 (125.5, 150.5) |
| Diastolic blood pressure, mmHg | 80.0 (73.0, 88.0) | 84.0 (76.0, 91.0) | 82.0 (75.5, 89.0) |
| Antihypertensive medication | |||
| Yes | 371 (3.6) | 20 093 (19.8) | 81 166 (21.5) |
| No | 10 002 (96.4) | 81 489 (80.2) | 296 484 (78.5) |
| Dementia prevalence, n (%) | |||
| All-cause dementia | 1125 (10.8) | 2443 (2.4) | 4565 (1.2) |
| Alzheimer’s dementia | 472 (4.6) | 1588 (1.6) | 2199 (0.6) |
| Vascular dementia | 137 (1.3) | 252 (0.2) | 1136 (0.3) |
| Vascular-related dementia | 908 (8.8) | 1289 (1.3) | 3279 (0.9) |
Values are reported as median (interquartile range) for continuous variables and number (percentage) for categorical variables.
CCHS, Copenhagen City Heart Study; CGPS, Copenhagen General Population Study.
aHigh alcohol intake was defined as >14/21 units per week for women/men (1 unit = 12 g alcohol, equivalent to one glass of wine or one beer (33 cL).
bSince the amount of alcohol intake in the UK Biobank has a substantial number of missing values and since this variable is not suitable for multiple imputation, we show the frequency of alcohol intake instead.
The results from individual cohorts were in the same direction across different exposures and outcomes. In the meta-analyses using random-effect models of fully adjusted observational Model 4 for all-cause dementia, the combined HRs ranged from 1.23 (1.13, 1.35) for myocardial infarction to 2.22 (1.41, 3.48) for stroke (Figure 2). Associations for Alzheimer’s disease and vascular dementia were similar to those for all-cause dementia; the effect sizes were smaller for Alzheimer’s disease and larger for vascular dementia. The largest HRs were for vascular dementia in individuals with stroke and ischaemic stroke, with pooled HRs of 5.68 (3.68, 8.75) and 5.34 (3.40, 8.39) (Figure 2). Hazard ratios from the age- and sex-adjusted and multifactorial-adjusted models did not differ materially (see Supplementary material online, Table S4). Between-study heterogeneities were observed for some analyses largely derived from the UK Biobank, and the results remained similar in both random- and fixed-effects models.
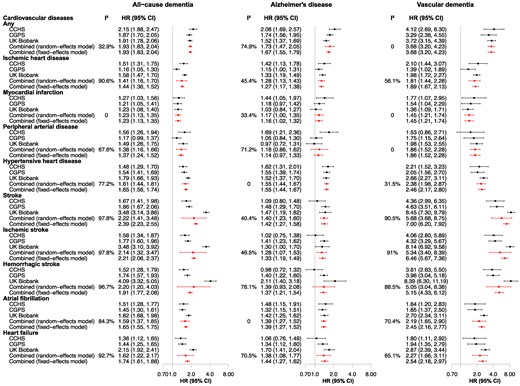
Observational associations between cardiovascular diseases and dementia from three cohorts. Estimates for each cohort were generated using cause-specific Cox regression models, adjusted for age (as time scale), sex, educational attainment, body mass index, smoking, physical activity, alcohol consumption, total cholesterol, cholesterol-lowering medication, and APOE genotype. The combined estimates were obtained through meta-analysing estimates from three cohorts using fixed-effects models. CCHS, Copenhagen City Heart Study; CGPS, Copenhagen General Population Study; CI, confidence interval; HR, hazard ratio.
In sensitivity analyses, results generally resembled those from the main analyses. The HRs were similar when stratified by sex (see Supplementary material online, Figures S1 and S2). Estimates showed similar trends by APOE ε4 or ε2 carrier status for all-cause dementia (see Supplementary material online, Figures S3 and S4), Alzheimer’s disease, and vascular dementia. Associations between CVDs and vascular-related dementia were analogous to those for vascular dementia; however, the HRs were attenuated (see Supplementary material online, Figure S5, left panel). Results were similar to the main results in meta-analyses after removing incident dementia cases occurring within 12 months after the ascertainment of CVDs (see Supplementary material online, Figure S6 compared with Figure 2). Results for mild cognitive impairment in the UK Biobank showed a similar trend as those for dementia (see Supplementary material online, Figure S7, left panel). Having multiple cardiovascular comorbidities (≥2 and ≥3 vs. ≤1) showed a stepwise decrease in the point estimates for the risk of dementia with increased comorbidities, possibly due to the changes towards healthier lifestyle and treatment of risk factors after CVD diagnosis (see Supplementary material online, Table S5).
Genetic study
Genetic instruments for CVDs explained variations of around 1.6% for heart failure to 14.1% for ischaemic heart disease, with sufficient strength (F statistics > 10) (see Supplementary material online, Table S2).
In the UK Biobank, the proportion of cases with CVDs increased stepwise from the lowest to the highest GRS quintiles (see Supplementary material online, Figure S8). Using all identified instruments (Model 1), genetically determined susceptibility for ischaemic heart disease (per logOR) was associated with risk of all-cause and vascular dementia (Figure 3), with ORs of 1.12 (1.02, 1.23) and 1.30 (1.08, 1.55). The associations remained for vascular dementia in Model 2 after removing shared genetic variants and in Model 3 after further excluding SNPs mapped to chromosome 19. The corresponding ORs were 1.24 (1.04, 1.50) and 1.24 (1.03, 1.50). Myocardial infarction showed similar trends as for ischaemic heart disease; however, the associations were not significant in both Model 2 and Model 3. Genetic susceptibility for stroke and ischaemic stroke were associated with all-cause dementia in all models, with ORs of 1.64 (1.35, 1.98) and 1.54 (1.28, 1.84) in Model 3. The associations for Alzheimer’s disease and vascular dementia followed similar patterns as for all-cause dementia, and the magnitudes of the effects were smaller for Alzheimer’s disease and larger for vascular dementia. After correcting for multiple testing, the associations in Model 3 for stroke and ischaemic stroke remained and the association between ischaemic heart disease and vascular dementia was borderline significant for (P-adjusted = 0.06) (see Supplementary material online, Table S6). No associations were observed for the remaining exposures. In sensitivity analyses, both stroke and ischaemic stroke were associated with risk of vascular-related dementia in all models, with corresponding ORs of 1.74 (1.39, 2.17) and 1.68 (1.36, 2.08) in Model 3 (see Supplementary material online, Figure S5, right panel); nevertheless, no significant association was detected for mild cognitive impairment, most likely due to a limited number of cases (see Supplementary material online, Figure S7, right panel).
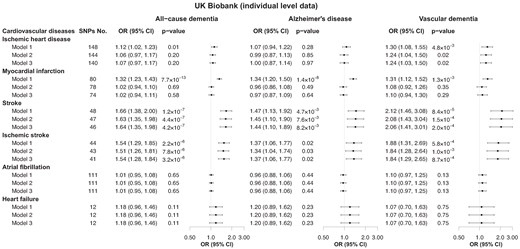
Genetic associations between cardiovascular diseases and dementia using individual-level data. Data presented were from the UK Biobank. Model 1 uses of all genome-wide significant independent single-nucleotide polymorphisms for each cardiovascular disease; Model 2 uses single-nucleotide polymorphisms from Model 1 excluding those significantly and directly associated with dementia at a stringent significance level (P < 1 × 10−5), i.e. excluding shared genetics; Model 3 uses single-nucleotide polymorphisms from Model 2 additionally excluding genetic variants mapped on chromosome 19 to fully account for the influence of the APOE gene. Details of the excluded single-nucleotide polymorphisms in each model are available in Supplementary material online, Table S3. CI, confidence interval; OR, odds ratio.
Results from two-sample MR in FinnGen are presented in Figure 4. Per logOR increase of genetic susceptibility for stroke, the OR was 1.21 (1.06, 1.38) for all-cause dementia using the inverse-variance weighted method with a similar association for ischaemic stroke [1.21 (1.06, 1.37)]. Associations between genetic susceptibility for stroke and ischaemic stroke and Alzheimer’s disease and vascular dementia were similar to those for all-cause dementia, and ORs for vascular dementia were the highest, with 1.91 (1.46, 2.49) for stroke and 1.67 (1.30, 2.14) for ischaemic stroke. Results from sensitivity analyses did not differ materially, and the associations for ischaemic stroke and stroke remained significant after correcting for multiple testing (see Supplementary material online, Table S7). Analyses further excluding SNPs mapped to chromosome 19 showed similar results (see Supplementary material online, Figure S9).
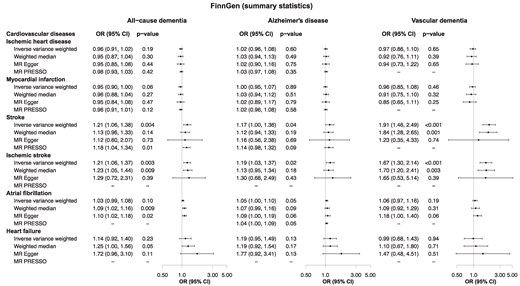
Genetic associations between cardiovascular diseases and dementia using summary statistics. Data were derived from the FinnGen study data release 9 (May 2023). CI, confidence interval; HR, hazard ratio.
Discussion
Our findings from three large prospective cohorts of ∼0.5 million individuals confirmed the known observational association between CVDs and dementia (graphic abstract). Furthermore, genetic studies supported a causal association between stroke and high risk of all-cause dementia, Alzheimer’s disease, and vascular dementia and between ischaemic heart disease and high risk of vascular dementia. These consistent observational and genetic findings suggest that CVDs are causal risk factors for dementia, supporting the importance of integrating CVD prevention into interventions for dementia risk reduction.
The associations between several CVDs and dementia have been established in prior observational studies. Several well-conducted meta-analyses have suggested stroke,36 non-stroke CVDs, and atherosclerosis37 as independent modifiable risk factors for dementia. A recent umbrella review including 25 meta-analyses showed that coronary heart disease, heart failure, and atrial fibrillation were associated with increased risk of all-cause dementia, while results were inconsistent for Alzheimer’s dementia.38 Moreover, previous studies have indicated that a high cardiovascular risk burden was associated with increased brain pathologies and dementia risk,39 whereas a favourable cardiovascular health score was associated with a lower risk of dementia.40 In line with these findings, our observational studies, combining three large homogenous cohorts with comprehensive analytic processes, confirmed the associations across a broader spectrum of CVDs and dementia subtypes, including vascular and vascular-related dementia that have only scarcely been touched upon. This underscores the robustness and generalizability of the observed relationships of CVDs and dementia.
Genetic studies to dissect causal aspects using MR designs have primarily focused on Alzheimer’s disease, which is the only dementia subtype with large genetic consortia data currently available. However, results from previous MR studies are controversial and sometimes counterintuitive. For example, while atrial fibrillation and stroke may not be associated with the risk of Alzheimer’s disease,23–25 heart failure may be associated with a lower or unchanged risk of Alzheimer’s disease.26–28 These inconsistencies could result from data source heterogeneities. More importantly, the analyses may suffer from methodological weaknesses, such as insufficiently addressing shared genetics and survival bias. Shared genetics is the situation where diseases may share a certain extent of genetic overlap such as variants that contribute to the pathologies and development of both diseases. This overlap might mask the underlying true causal association if not properly addressed. Twin studies could remove the shared genetic background, and in a Swedish observational twin study, cardiometabolic multimorbidity (encompassing diabetes, heart disease, and stroke) was associated with increased risk of dementia in dizygotic but not in monozygotic twins (OR 1.15 vs. 0.99).12 This suggests that the observed associations were partly explained by the shared genetic factors between CVDs and dementia. However, the analysis was constrained by a limited sample of monozygotic pairs, comprising 54 pairs as opposed to 302 dizygotic pairs, coupled with a modest number of stroke incidents, which collectively could attenuate the statistical power. In contrast, consortia GWAS data did not identify shared genetic factors between ischaemic stroke and Alzheimer’s disease.41 This supports our present findings that genetic associations between stroke and dementia are not fully explained by shared genetic factors. The associations between ischaemic heart disease and Alzheimer’s disease disappeared after removing APOE-related instruments, indicating that these associations were driven by shared APOE aetiology. Additionally, two-sample MR studies using GWAS from case-control designs on dementia might suffer from survival bias derived from selective survival on the exposure, meaning that participants should have survived genetically high levels of cardiovascular risk factors and high odds of CVDs and live long enough to be recruited. Particularly, the updated 2024 report of the Lancet Commission for dementia prevention, intervention, and care discussed that stroke and dementia share risk factors of less education, infrequent exercise, hypertension, heart disease, and social isolation, but some people with these risk factors will not develop dementia because they die at a young age before dementia develops.2 With the acknowledgment of survival bias, our two-sample MRs were conducted based on the nested case-control GWAS from the prospective FinnGen study and confirmed the possible causal role of stroke and ischaemic stroke for dementia. To our knowledge, no relevant MR studies on other types of dementia have ever been done, possibly because of the lack of publicly available genomic consortia. Interestingly, ischaemic heart disease was associated with vascular dementia after stringent exclusion of APOE in the UK Biobank, despite not being confirmed by the two-sample MR using summary statistics from the FinnGen study. Further powerful studies with more vascular dementia cases to validate our findings are warranted.
The associations between CVDs and dementia pathologies involve various biological mechanisms.8,9 For stroke, the most widely recognized putative neuropathological substrates and cerebral vulnerability are the functional and structural alterations of the vasculature, leading to chronic cerebral hypoperfusion and hypoxia.42 These changes may cause neurovascular dysfunction characterized by endothelial dysfunction, which can result in compromised integrity of the blood–brain barrier, enhancing permeability. This further impairs amyloid-beta clearance in the brain,43 as well as activates immune responses through extravasation of neurotoxic blood-derived antigens.44 Such non-resolving neuroinflammation may form a vicious circle, in turn triggering an increased risk of cerebrovascular events and increased infarct size.44 Moreover, the severe white matter changes and medial temporal lobe atrophy, both as pre-existing pathology and as sequelae after ischaemic brain injury, may contribute to cortical grey matter thinning and therefore increase the risk of cognitive decline.45 In contrast, ischaemic heart disease predominantly contributes to dementia through systemic mechanisms. Chronic myocardial ischaemia is closely linked to systemic inflammation, oxidative stress, and metabolic dysregulation and leads to reduced cardiac output and cerebral hypoperfusion, all of which increase susceptibility to vasculature pathologies, potentially exacerbating global neurodegenerative processes. In addition, GWAS have identified multiple variants that are jointly associated with cardiovascular risk factors and Alzheimer’s disease, such as plasma lipids.5 Therefore, the cumulative burden of cardiovascular risk factors that result in CVDs could also play a role in the development of dementia. Importantly, while stroke tends to have direct and localized effects on brain structure and function, ischaemic heart disease contribution may be more diffuse, mediated by systemic factors and cumulative vascular burden. Together, these mechanisms work in concert synergistically or additively, amplifying their deleterious effects on the brain, ultimately leading to substantial cognitive impairment and thus dementia. These distinctions also underscore the need for tailored prevention strategies, focusing on cerebrovascular health for stroke survivors and systemic risk management in patients with ischaemic heart disease.
This study has several strengths. Our observational study benefits from large sample sizes from three cohorts with long-term follow-up, high-quality disease ascertainment, comprehensive confounding information, and the opportunity to distinguish between different dementia subtypes. Furthermore, we triangulated our findings from different study designs and applied multiple statistical approaches. The results reached the same conclusion, strengthening the reliability. Our study also has several limitations. Firstly, disease documentation in the Copenhagen cohorts was hospital-based clinical diagnoses retrieved from the Danish registries. While it ensures the completeness of data, misclassification may occur. Nevertheless, dementia diagnosis in Denmark is diagnosed based on information from neurologic tests, cerebrospinal biomarkers, imaging, and clinical symptoms and has been demonstrated with high validity.46 In the UK Biobank, the response rate is low (5% response rate vs. 43–70% in the Copenhagen cohorts) which may lead to a healthy participant bias. In addition, parts of the diagnoses of dementia are from primary care, which is subject to a range of potential biases and fluctuations over time due to national and local policy initiatives and local processes and procedures. This may jointly explain that most of the observational estimates from the UK Biobank are larger than those from the Copenhagen cohorts. Secondly, we are not able to further dissect the effect of stroke lesion burden/location (severity, recurrent stroke, volume of infarcts) on dementia due to lack of this kind of data. Lastly, our findings are specific to White individuals of European ancestry, and thus, the generalizability to populations with different ethnicities is limited.
Conclusion
Our study provides evidence that associations between stroke and increased risk of all-cause dementia, Alzheimer’s disease, and vascular dementia are highly likely to be of a causal nature. Further, we also show associations between ischaemic heart disease and later development of vascular dementia. Collectively, these findings highlight the importance of integrating CVD prevention and management strategies into interventions to potentially reduce dementia risk.
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology.
Acknowledgements
We thank the participants and staff of the Copenhagen City Heart Study and the Copenhagen General Population Study for their important contributions. This research has been conducted using the UK Biobank Resource under Application Number 66214, and this work uses data provided by patients and collected by the NHS as part of their care and support. We thank the FinnGen study for making their summary statistics available.
Author contribution
J.L., J.Q.T., and R.F.-S contributed to the study conception and design, accessed, and verified the data. J.L., I.J.R., and J.Q.T. performed the statistical analysis. J.L. and R.F.-S. drafted the manuscript. All authors were involved in data acquisition, analysis, or interpretation, and contributed with important intellectual content to the critical revision of the manuscript. A.T.-H., B.G.N., and R.F.-S. obtained funding for the study. R.F.-S. supervised the study. JL, IJR, JQT and RFS had full access to all the data in the study. BGN and ATH had full access to data in the CCHS and CGPS cohorts. All authors had final responsibility for the decision to submit for publication.
Funding
The study was supported by grants from the Lundbeck Foundation (R278-2018-804), the Danish Heart Foundation, Innovation Fund Denmark (9084-00020B), and the Research Fund at Sygeforsikringen Danmark, all to R.F.-S. J.L. was supported by a BRIDGE programme post doc fellowship from the Novo Nordisk Foundation.
Data availability
Data from the Copenhagen cohorts are available to the corresponding author R.F.-S. upon reasonable request. Data from the UK Biobank are available upon application. Data from FinnGen study are available on the website https://www.finngen.fi/.
References
Author notes
Conflict of interest: J.L., I.J.R., and J.Q.T. have nothing to declare. B.G.N. reports consultancies or talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. A.T.-H. reports consultancies or talks sponsored by Amgen, Akcea, AstraZeneca, Draupnir Bio, Novartis, Regeneron, Sanofi, and Silence Therapeutics. R.F.-S. reports consultancies sponsored by Novo Nordisk.



Comments