-
PDF
- Split View
-
Views
-
Cite
Cite
Dalil Sadki, Sami Fawaz, Jean-Sebastien Liegey, Yann Pucheu, Romain Boulestreau, Gauthier Beuque, Jeanne Foucher, Louise Hein, Thierry Couffinhal, Differential cardiovascular impacts of sodium salts: unveiling the distinct roles of sodium chloride and sodium bicarbonate—consequences for heart failure patients, European Journal of Preventive Cardiology, 2025;, zwaf020, https://doi.org/10.1093/eurjpc/zwaf020
Close - Share Icon Share
Abstract
Misconceptions surrounding sodium compounds, particularly the interchangeable use of sodium and sodium chloride (table salt), persist within the medical community, influencing dietary recommendations and patient management especially in heart failure (HF) patients with chronic kidney disease (CKD). This narrative review aims to dissect these misconceptions and discusses the physiological impacts of sodium, chloride, and sodium bicarbonate on cardiovascular (CV) physiology. The conflation of sodium and sodium chloride in dietary recommendations has obscured critical differences in their physiological effects. While sodium chloride is traditionally linked to hypertension, emerging evidence suggests that chloride, rather than sodium, may be the primary driver of hypertension and activation of the renin-angiotensin-aldosterone system. In contrast, sodium bicarbonate, when administered orally, seems to exert minimal effects on blood pressure and plasma volume, offering a promising and safe way for managing HF patients with renal insufficiency. Indeed, the therapeutic benefits of sodium bicarbonate in CKD patients, including preservation of muscle mass, slowing of renal function decline, lowering of all-cause mortality, and improved nutritional status, are quite proven; this underscores its potential utility in patients suffering from both HF and renal insufficiency. Despite concerns about metabolic alkalosis, recent studies suggest that judicious sodium bicarbonate therapy may mitigate major adverse cardiac events without exacerbating HF. This review advocates for a paradigm shift in CV medicine, urging clinicians to discern between sodium chloride and other sodium salts, particularly sodium bicarbonate, in patient care. By elucidating these distinctions, clinicians can tailor dietary recommendations and therapeutic interventions to optimize outcomes for HF patients with CKD and address the multi-faceted complexities of atherosclerotic disease.
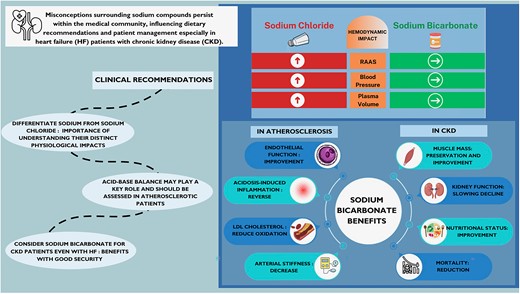
NaCl, sodium chloride; NaHCO3, sodium bicarbonate; HF, heart failure; CKD, chronic kidney disease; RAAS, renin angiotensin aldosterone system.
Lay Summary
This review clarifies the physiological differences between sodium chloride and sodium bicarbonate, advocating for a nuanced approach in managing cardiovascular (CV) health in patients with heart failure (HF) and chronic kidney disease (CKD).
Sodium chloride (table salt) is commonly associated with hypertension, whereas chloride, rather than sodium itself, appears to be the primary factor influencing blood pressure and activation of the renin-angiotensin-aldosterone system.
Sodium bicarbonate shows promise in managing CKD by preserving muscle mass, slowing renal decline, reducing all-cause mortality, and improving nutritional status without significantly impacting blood pressure or plasma volume, making it secure in patients with HF.
By distinguishing between these sodium compounds, clinicians can better tailor dietary recommendations and treatments to improve outcomes for patients with HF and CKD, advancing the management of CV diseases.
Introduction
Misconceptions about sodium compounds
The confusion between sodium and sodium chloride (table salt) is widespread within the medical scientific community, which often uses these terms interchangeably without distinction. This confusion is compounded by dietary classifications based on sodium content rather than sodium chloride content and by the varying effects of sodium when it is combined with chloride vs. when it is combined with other ions, such as bicarbonate.
Consequently, foods and beverages high in sodium bicarbonate are often prohibited for patients with heart failure (HF), despite potential benefits. This is particularly notable given that patients with HF frequently exhibit renal insufficiency. Renal insufficiency in chronic kidney disease (CKD) leads to metabolic acidosis that is responsible for numerous complications musculoskeletal, renal, and endocrine damage and increases overall mortality. The benefits of bicarbonates in patients with renal insufficiency are well established.1–7
Given the close link between renal and HF—nearly 50% of patients with HF also suffer from renal insufficiency—it is crucial to reconsider the advisability of recommending dietary sodium restrictions for these patients. This is especially important considering the potential burden of stringent and potentially painful, dietary rules, regardless of whether the sodium is in the form of sodium chloride or other compounds.8,9
Methods
We conducted a narrative review focused on the physiological impacts of sodium, chloride, and sodium bicarbonate on cardiovascular (CV) physiology, addressing selected topics. The literature search was carried out using the Google Scholar and PubMed databases to identify articles in English published between November 1990 and November 2023. Search terms included ‘bicarbonate’, ‘heart failure (HF)’, ‘renin-angiotensin system (RAS)’, and ‘acidemia’. Abstracts were screened by two authors (D.S. and T.C.) to assess their relevance, with any discrepancies resolved by a third author (S.F.). Priority was given to recent randomized controlled trials and meta-analyses. Additional articles were included if they were deemed relevant within the references of the selected articles. In total, we reviewed more than 100 abstracts, selecting 41 original research studies and 4 meta-analyses for inclusion in this review.
Results and discussion
The longstanding misunderstanding of sodium chloride and sodium bicarbonate in CV medicine has led to potentially sub-optimal management of patients with HF, namely those with CKD. While both compounds contain sodium, their distinct physiological impacts necessitate different considerations in clinical practice. It is crucial to differentiate these effects to optimize patient outcomes, particularly in populations where sodium balance is critically linked to disease management and prognosis.
Confusion among sodium, sodium chloride, and sodium bicarbonate
This paragraph aims to clarify the distinctions between different forms of sodium, elaborates on their uses, and explains the legal and health implications of misleading labelling.
Dietary guidelines for patients with HF largely revolve around a low sodium chloride (NaCl) or table salt diet. Patients with HF are instructed not to add salt during cooking, to avoid adding NaCl during meals, and to drastically reduce or eliminate consumption of certain highly salted industrial products.
Healthcare professionals—including cardiologists, nurses, and dietitians—emphasize the critical need to monitor dietary sodium. They routinely advise patients to review nutrition labels for sodium content in both food and mineral or bottled waters. However, understanding these labels can be challenging since sodium frequently appears as an ingredient. Recognizing the two primary forms of dietary sodium—table salt (sodium chloride) and sodium bicarbonate—is essential for effective dietary management.
Table salt, which consists of both sodium and chloride, contains ∼400 mg of sodium per gram. This means that sodium comprises roughly 40% of table salt, with the remainder being chloride. On the other hand, sodium bicarbonate, or baking soda, consists of sodium linked to a bicarbonate group formed from hydrogen, carbon, and oxygen molecules; in 1 g of sodium bicarbonate, there are ∼274 mg of sodium.
Table salt serves multiple roles in food production, such as curing meat, baking, thickening, retaining moisture, enhancing flavour, and preserving food. In the food industry, sodium bicarbonate is primarily used as a leavening agent in baked goods. It reacts with acidic ingredients to release carbon dioxide, aiding in dough rise and texture formation. Additionally, sodium bicarbonate plays roles in pH regulation and flavour enhancement and is even a component in effervescent beverages. In mineral waters, sodium bicarbonate is often added or naturally present, serving to improve taste, provide buffering properties, and help resist pH changes when other substances are introduced.
However, the classification of food and water products typically focuses on total sodium content rather than distinguishing between sodium chloride and sodium bicarbonate. This generalization can overlook how different sodium compounds contribute uniquely to health. For instance, healthcare providers caution against consuming high-sodium waters, such as Saint-Yorre and Vichy Célestins. Despite their high sodium content, these waters are low in chloride and rich in bicarbonate, leading to unique health implications. The sodium in these waters is primarily chelated by bicarbonate, not chloride, resulting in a sodium chloride concentration in Vichy waters comparable with that of tap water, albeit with a significantly higher bicarbonate content.
Haemodynamic impact: comparative effects of sodium chloride and bicarbonate on blood pressure and renin activity
Over the past century, the concept of sodium balance and its haemodynamic impact, particularly in hypertension, have been extensively studied. It is firmly established that excessive intake of table salt is correlated with hypertension, although there is significant inter-individual variability in the strength of this correlation, and hypertension is independently correlated with HF.10 This association has been immediately and tacitly attributed to sodium itself. However, this is not scientifically founded, as numerous experimental studies from the 20th century have shown.
Effects on the renin-angiotensin-aldosterone system
The renin-angiotensin-aldosterone system (RAAS) is considered a central element in the regulation of blood pressure. The primary factor regulating renin secretion is generally believed to be the concentration of sodium in the distal convoluted tubule, via the macula densa.11,12 This regulation operates through a feedback mechanism: a decrease in sodium concentration at the macula densa leads to increased renin secretion and vice versa.13 In the 1970s and 1980s, several studies investigated the evolution of renin concentration in the blood of rats that had previously received supplementation with a chloride salt varying the associated cation or a sodium salt varying the associated anion.11,14,15 Whether administered intravenously or orally, these studies tend to show that renin concentration is significantly inhibited by the presence of chloride, independent of sodium intake.16
Similar findings have been observed in humans, with significant inhibition of plasma renin activity during i.v. administration of sodium chloride, which is not seen with the administration of sodium bicarbonate.17 Therefore, sodium does not appear to be the determining factor in the regulation of renin secretion at the macula densa, but rather the chloride is associated with sodium in sodium chloride.16 This suggests that the RAAS response might be more influenced by the presence of chloride than by sodium alone.18
Direct effects on blood pressure
The Dahl rat is an experimental model of hypertension sensitive to salt. In this model, it has been shown that the blood pressure of rats supplemented with sodium chloride was significantly higher after 5 weeks compared with rats supplemented with sodium bicarbonate. In the latter group, there was no significant difference compared with the control group, which was fed a low sodium chloride diet.14
These observations in rats have been corroborated by other trials.19 In humans, several small-scale experimental studies have highlighted that the administration of sodium salt with bicarbonate or potassium does not cause the hypertension induced by the administration of sodium salt with chloride.20–25 A small, double-blind, randomized controlled trial in elderly hypertensive subjects showed that consumption of water rich in sodium bicarbonate did not increase blood pressure compared with a low-salt diet.24 More broadly, in the numerous studies assessing sodium bicarbonate in patients with renal insufficiency, no significant increase in blood pressure was observed in patients treated with bicarbonate.3–5,26–30 A meta-analysis published in 2023, which included 14 randomized controlled trials, found no influence of oral sodium bicarbonate on blood pressure in patients with CKD.29
Effects on plasma and interstitial volume
Regarding the effect on plasma volume, studies in rats have shown that it is significantly increased by the intake of sodium chloride, but not by the intake of sodium alone.31 In both humans and rats, compared with sodium chloride, sodium bicarbonate appears to cause a similar weight gain, yet without an increase in plasma volume.25 The most plausible hypothesis to explain this apparent paradox between weight gain and the absence of plasma volume increase is a change in sodium distribution.32–34
However, it should be noted that, compared with placebo, in patients with renal insufficiency, several trials studying sodium bicarbonate report no significant weight gain.1,3,27,35 This suggests that the effects of sodium bicarbonate on body weight and fluid distribution may differ significantly from those of sodium chloride, possibly due to differences in how these compounds interact with body fluids and tissues.
Cardiovascular impact of sodium bicarbonate
Metabolic acidosis in chronic kidney disease
Metabolic acidosis is a common complication in patients with CKD, particularly when the glomerular filtration rate drops below 30 mL/min per 1.73 m2. The primary pathogenesis of metabolic acidosis in CKD involves impaired excretion of non-volatile acids, mainly ammonium, along with diminished bicarbonate reabsorption and production. Failure to neutralize the net endogenous acid load leads to acid retention. Metabolic acidosis is generally defined when serum bicarbonate levels fall below 22 mmol/L. While the exact glomerular filtration rate (GFR) threshold for developing metabolic acidosis is not well defined, its prevalence increases as kidney function deteriorates.
Metabolic acidosis alters heart and blood vessel function and can lead to arrhythmias, bone demineralization, muscle weakness, and wasting and accelerates the progression of CKD.
These complications worsen with the decline in renal function but are present even before the onset of overt metabolic acidosis, with pH maintained within normal limits by compensatory mechanisms that are detrimental in the long term.36,37
The benefits of bicarbonates for patients with renal insufficiency are well documented; oral sodium bicarbonate supplementation, particularly in cases of acidosis, helps preserve muscle mass, slow renal function decline, improve nutritional status, preserve bone mass, and even reduce mortality.1–7
Safety profile in patients with heart failure
Most trials studying the effect of oral sodium bicarbonate have been conducted in patients with renal insufficiency and excluded those with symptomatic or congestive HF. The confusion between sodium and sodium chloride was further reinforced by the results of an observational study (based on the CRIC cohort, nearly 4000 patients) examining the level of blood bicarbonate in patients with CKD.38 In this study, there appeared to be an increased risk of HF events correlated with higher blood bicarbonate levels. However, this was observed only at abnormally high bicarbonate levels, in patients with metabolic alkalosis, a condition well known to be detrimental to cardiac function.39
This risk in a state of alkalosis has been observed subsequently but does not exist for bicarbonate levels remaining below 26 mM. Thus, while sodium bicarbonate therapy can be safe for patients with HF when carefully monitored, caution is warranted when approaching the upper limits of normal blood bicarbonate levels to avoid the adverse effects associated with metabolic alkalosis.35,40–43
Recently, a study conducted on 25 599 patients from a multi-institutional electronic medical record database in Taiwan evaluated the effect of oral sodium bicarbonate use on major adverse cardiac events (MACEs) and mortality in patients with end-stage CKD prior to the dialysis stage.35 This study is the first that did not exclude patients with HF, even those in New York Heart Association Class III and IV. It is also the first study evaluating oral sodium bicarbonate in CKD that uses MACE as the primary outcome measure. The study showed that there was no increased risk of acute HF found [hazard ratio (HR): 0.92, 95% confidence interval (CI) 0.88–0.96, P < 0.001], and the all-cause mortality was significantly lower in oral sodium bicarbonate users compared with sodium bicarbonate non-users (HR: 0.75, 95% CI 0.74–0.77, P < 0.001). However, this retrospective cohort study does not provide a high level of evidence, though it remains the only one of its kind. Further studies, particularly prospective ones, potentially in the form of randomized controlled trials, are required to formally confirm the safety of oral sodium bicarbonate in this indication, as well as its potential to reduce overall mortality, particularly through its impact on kidney failure.
Effects of sodium bicarbonate on atherosclerotic disease
The relationship between sodium bicarbonate supplementation and atherosclerosis is not well understood. In the CRIC cohort, no association was found between bicarbonate levels and CV events related to atherosclerosis.38 However, in the Fremantle Diabetes Study of 1283 diabetic patients followed for 12 years, serum bicarbonate was independently and negatively associated with incident coronary heart disease, but there were no independent associations between serum bicarbonate and all-cause mortality or incident HF.41 Additionally, it has been shown in hypertensive patients that a bicarbonate level <22 mM is associated with an increase in acute CV events (acute coronary syndrome, stroke, and CV death).44
The study discussed in the previous section,35 which evaluates the effect of oral sodium bicarbonate on MACEs in a population of patients with HF and kidney failure, showed that the use of sodium bicarbonate significantly reduces MACE (HR: 0.95, 95% CI 0.92–0.98, P < 0.001), particularly the risk of myocardial infarction and stroke, without altering the risk of dialysis.
Patients with CKD develop metabolic acidosis as they approach Stages 3 and 4, during which accelerated atherogenesis may occur. Indeed, acidosis induces a state of low-grade inflammation, endothelial dysfunction, and increased arterial stiffness, leading to accelerated atherosclerosis.44–51 Correcting this acidosis has been shown to improve endothelial function and reduce oxidation of LDL cholesterol, thereby slowing the progression of atherosclerosis.43,52,53 Sodium bicarbonate plays a role in re-establishing acid–base balance. The positive impact of sodium bicarbonate can also be achieved through other methods that promote alkalization, such as a diet rich in fruits and vegetables, as demonstrated in a comparative study.54 However, it is necessary to remain cautious of the deleterious effects of metabolic alkalosis, particularly on cardiac function.42,55
Dietary recommendations and cardiovascular health
The European Society of Cardiology guidelines and the American Heart Association Dietary Guidance provide detailed recommendations on managing sodium intake in patients with CV disease, CKD, and HF.56,57
High intake of salt is some of the main determinants for elevated blood and could be targets for interventions to reduce blood pressure for the primary prevention of HF.10
For the general population and patients with CV risk, sodium intake should be limited to <5 g of salt per day (∼2 g of sodium). This reduction is particularly emphasized in patients with hypertension to lower blood pressure and decrease CV risks. Patients are encouraged to prepare foods with little or no added salt and to reduce consumption of processed foods that are high in sodium.
For patients with HF, a stricter sodium restriction is recommended to help control fluid retention and alleviate symptoms. Sodium intake should ideally be restricted to <1.5 g per day to reduce volume overload and improve clinical outcomes.
In patients with CKD, sodium restriction plays a crucial role in managing hypertension and slowing the progression of renal damage. To effectively manage CV risk factors, sodium intake should be limited to <2 g per day.
Potassium chloride is often recommended as an alternative to sodium chloride to aid in reducing sodium consumption and managing hypertension. Increasing dietary potassium through potassium-rich foods, such as fruits and vegetables, is encouraged, as it helps balance sodium’s hypertensive effects, thereby offering a protective role in CV health.58,59 However, caution is necessary in patients with impaired kidney function to avoid hyperkalaemia, which can be a significant concern.
Oral sodium bicarbonate is not specifically mentioned as a recommendation for CV prevention in the reviewed guidelines. In patients with CKD, acid–base balance correction using sodium bicarbonate is already recommended by nephrology societies to reduce mortality and slow the decline of kidney function.60
Based on this review, we would suggest, in future dietary recommendations, a need for further differentiation between sodium chloride (common salt) and other forms of sodium, such as sodium bicarbonate. Future guidelines may specifically address the different CV impacts of oral sodium chloride vs. oral sodium bicarbonate, encouraging dietary classifications that focus on the specific sodium compound rather than on total sodium content. For instance, oral sodium bicarbonate may offer benefits in managing kidney disease and HF, with evidence suggesting a favourable safety profile in patients with atherosclerotic disease. Moreover, replacing sodium chloride with potassium chloride or using oral sodium bicarbonate under medical supervision could be beneficial strategies in CV care, particularly for patients with comorbid CKD and HF. These evolving perspectives may lead to more targeted and effective dietary guidelines, optimizing CV outcomes in vulnerable populations.
Conclusions
In CV medicine, sodium chloride and sodium bicarbonate are often assumed to have similar clinical consequences due to their common component, sodium. However, extensive studies in both animal models and humans have consistently highlighted distinct physiological effects between sodium chloride and other sodium salts, such as sodium bicarbonate, with particularly notable differences in their respective impacts on blood pressure regulation and plasma volume expansion.
The findings presented here pertain exclusively to oral sodium bicarbonate, rather than to i.v. administration, underscoring the necessity for clinicians to recognize these distinctions in treatment contexts. Furthermore, this review advocates a paradigm shift in patient management, emphasizing the need to reconsider recommendations for sodium restriction, particularly in patients with HF and renal insufficiency, as well as those with atherosclerotic vascular disease and renal impairment. There is accumulating evidence that supports the safety and potential benefits of oral sodium bicarbonate in these populations, even if we recognize that the level of evidence is not high. This underscores a need for further rigorous studies to confirm the therapeutic role of oral sodium bicarbonate in CV and renal management, especially in patients at heightened cardiovascular risk.
Acknowledgements
We thank the medical team of the ‘Service des Maladies Coronaires et Vasculaires, CHU de Bordeaux’, for their support and constructive criticism. Special thanks to Sakina Abbadi for her invaluable assistance in creating the graphical abstract.
Author contribution
D.S. originated the topic idea and conducted the literature review. D.S. and T.C. wrote and edited the paper. S.F., J.-S.L., Y.P., R.B., G.B., J.F., and L.H. reviewed and commented on the manuscript. All authors approved the final version of the manuscript.
Funding
This study was supported by the Société Française de Cardiologie and the Fédération Française de Cardiologie.
Data availability
No new data were generated or analysed in support of this research.
References
Author notes
Conflict of interest: none declared.



Comments