-
PDF
- Split View
-
Views
-
Cite
Cite
Naoko P Kato, Marie Mattisson, Pernilla Grahn, Maria Liljeroos, Peter Johansson, Anna Strömberg, Tiny Jaarsma, Describing the use of remote dielectric sensing and handheld ultrasound in assessing lung congestion in heart failure patients within a primary care setting, European Journal of Cardiovascular Nursing, Volume 24, Issue 2, March 2025, Pages 325–331, https://doi.org/10.1093/eurjcn/zvae157
Close - Share Icon Share
Abstract
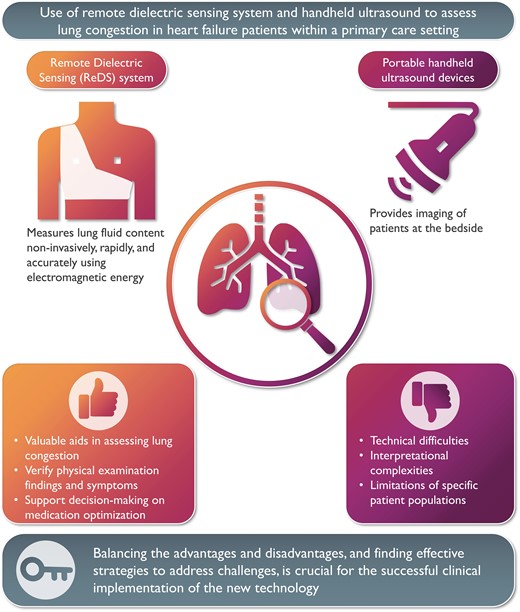
Thorough consideration of user experiences and the weighing of advantages and disadvantages are essential when implementing new technology in clinical practice. This article describes a primary care nurse’s experience using two technologies to monitor lung congestion in six patient cases: a remote dielectric sensing device for non-invasive lung fluid measurement and a portable handheld ultrasound device. Both can support decision-making when assessing lung congestion in heart failure patients. However, technical difficulties and interpretational complexities are inherent in their use. Balancing these advantages and disadvantages and finding effective strategies to address challenges is crucial for successful clinical implementation.
Remote dielectric sensing technology and handheld ultrasound devices are valuable aids for the primary care nurse in assessing lung congestion in heart failure patients and optimizing heart failure medication.
Several challenges, including technical difficulties, interpretational complexities, and the limitations of specific patient populations, are important to note when using new technologies.
Weighing the pros and cons of adopting new technologies and finding potential strategies to address them is an important step towards their effective implementation in various environments.
Introduction
There has been a growing emphasis on accurately assessing lung congestion to prevent hospitalizations for heart failure (HF),1,2 since increases in lung fluid content are among the earliest physiological changes in the progression of worsening HF, whereas the signs and symptoms of HF typically appear later. Conventional methods for assessing lung congestion, such as physical examination and symptom monitoring, have been shown to be insensitive markers.3,4 Several new digital health technologies for monitoring lung congestion have been developed for patients with HF.2 In our study, we focused on the two devices. First is a novel device using remote dielectric sensing technology (ReDS, Sensible Medical Innovations Ltd) designed to measure lung fluid content based on electromagnetic energy (see Figure 1).5 The ReDS system uses two sensors placed on the front and back of the patient’s thorax within a wearable vest for a 90 s measurement. Its accuracy in quantifying lung fluid content has been validated against chest computerized tomography scan6 and invasive haemodynamic measurements.7 Normal intrathoracic fluid ranges from 20 to 35%. Readings above 35% indicate increased fluid content, while those below 20% suggest volume depletion. The ReDS system is available in the European Union, the USA, and other regions.3,8 Recent studies confirmed the effectiveness of ReDS-guided HF management in preventing HF readmission.8,9 And second, portable handheld ultrasound devices (GE Healthcare, V-scan; Figure 1) are designed for bedside use to enhance and extend physical examinations. Their use has increasingly been used in cardiac clinics and emergency departments.10,11
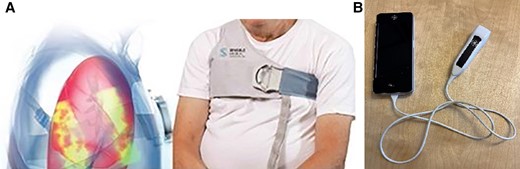
(A) Remote dielectric sensing technology (Sensible Medical Innovations Ltd, Netanya, Israel) assesses lung congestion using electromagnetic-based technology. (B) Handheld ultrasound, V-scan (GE Healthcare).
There is increased attention on HF management in primary care.12–14 These new digital health technologies may serve as alternatives or complements to cardiac specialist knowledge for healthcare professionals. However, new technology implementation in clinical practice is not always successful, primarily due to gaps between research environments and real clinical settings. To ensure successful implementation, it is crucial to thoroughly consider user experiences and weigh the pros and cons of adopting new technologies. In this paper, we described the experiences of a primary care nurse in utilizing two devices in six patient cases and discussed the additional value of the information obtained from these devices (see Central Illustration).
Training for new technology
A primary care nurse with experience in inpatient cardiology attended a 1 h lecture given by a HF nurse specialist. The specialist nurse, who had extensive experience with V-scan and HF, covered how to understand and interpret findings such as comet tail artefacts, pleural effusion, and enlarged inferior vena cava (IVC). Following the lecture, the primary care nurse underwent V-scan training with 10 HF patients in primary care, under the supervision of another HF nurse specialist skilled in using the V-scan to assess lung congestion. For the ReDS, the same primary care nurse underwent a half-day training session, which included watching a tutorial video and receiving instructions from a company instructor, followed by five practice sessions with nursing colleagues. After the training, the primary care nurse used V-scan and ReDS on six HF patients at two or three distinct time points (Table 1), who were then scheduled for regular follow-up visits at a nurse-led clinic in a primary care centre. This study was approved by the Swedish ethics committee (2023-03180-01), and informed consent was obtained from all study participants.
Experiences with new technology
Patient 1
A 75-year-old man diagnosed with HF (NYHA III) showed elevated ReDS values (>45), indicating hypervolemic status, and pleural effusion was identified using V-scan during the first and second follow-up visits, as depicted in Figure 2. Additionally, the patient’s IVC measurement exceeded 21 mm with a collapsibility index below 50%. The nurse found this V-scan data to be informative, as it confirmed her physical examination findings of lung auscultation through visual confirmation of pleural effusion. On the other hand, the nurse did not detect B-lines using V-scan during any encounters (Table 2), leading to feelings of uncertainty and scepticism regarding the ReDS values of ≥45 due to the patient’s obesity and her limited familiarity with the device. This uncertainty prompted the nurse to question the trustworthiness of both devices, posing the dilemma of ‘which device to trust?’ In this case, both ReDS and V-scan and physical examination suggested pulmonary congestion. However, the nurse expressed feelings of uncertainty and scepticism regarding the ReDS measurements due to limited experience and technical concerns. The nurse placed greater trust in the results from V-scan, e.g. the absence of B-lines.
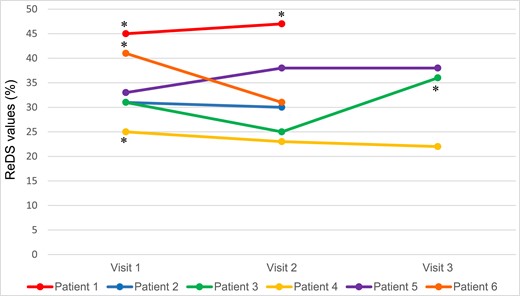
Changes in remote dielectric sensing values and results obtained by handheld ultrasound V-scan. The presence of pulmonary congestion was determined based on remote dielectric sensing values exceeding 35%. For the V-scan, this was defined as either (A) inferior vena cava measurement greater than 21 mm with a collapsibility index below 50%, (B) the presence of more than three B-lines, or (C) the presence of pleural effusion. *The presence of pulmonary congestion assessed by a handheld ultrasound.
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . |
|---|---|---|---|---|---|---|
| Age | 75 | 78 | 87 | 86 | 88 | 93 |
| Gender | Male | Male | Female | Female | Female | Female |
| Living situation | Living alone | Living together | Living together | Living alone | Living alone | Living alone |
| Aetiology of HF | Hypertension arrhythmia | Hypertension arrhythmia | Ischaemic disease | Hypertension | Persistent foramen ovale | Ischaemic disease |
| NYHA class, III | III | III | II | III | III | II |
| NT-proBNP (pg/mL) baseline | 4922 | 893 | 399 | 877 | 5390 | 2951 |
| Types of HF | HFmrEF | — | HFpEF | HFmrEF | HFmrEF | HFpEF |
| Body mass index | 27.6 | 37.0 | — | 27.3 | — | 28.2 |
| Height, cm | 170 | 185 | — | 148 | — | 161 |
| Weight, kg | 79.7 | 126.5 | 65.6 | 59.8 | 98.8 | 73 |
| Chronic obstructive pulmonary disease /asthma | No | Yes | No | Yes | Yes | Yes |
| Diabetes | Yes, type 2 | Yes, type 1 | No | No | No | Yes, type 2 |
| Renal failure | Yes | No | No | No | Yes | No |
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . |
|---|---|---|---|---|---|---|
| Age | 75 | 78 | 87 | 86 | 88 | 93 |
| Gender | Male | Male | Female | Female | Female | Female |
| Living situation | Living alone | Living together | Living together | Living alone | Living alone | Living alone |
| Aetiology of HF | Hypertension arrhythmia | Hypertension arrhythmia | Ischaemic disease | Hypertension | Persistent foramen ovale | Ischaemic disease |
| NYHA class, III | III | III | II | III | III | II |
| NT-proBNP (pg/mL) baseline | 4922 | 893 | 399 | 877 | 5390 | 2951 |
| Types of HF | HFmrEF | — | HFpEF | HFmrEF | HFmrEF | HFpEF |
| Body mass index | 27.6 | 37.0 | — | 27.3 | — | 28.2 |
| Height, cm | 170 | 185 | — | 148 | — | 161 |
| Weight, kg | 79.7 | 126.5 | 65.6 | 59.8 | 98.8 | 73 |
| Chronic obstructive pulmonary disease /asthma | No | Yes | No | Yes | Yes | Yes |
| Diabetes | Yes, type 2 | Yes, type 1 | No | No | No | Yes, type 2 |
| Renal failure | Yes | No | No | No | Yes | No |
HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; NYHA, New York Heart Association.
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . |
|---|---|---|---|---|---|---|
| Age | 75 | 78 | 87 | 86 | 88 | 93 |
| Gender | Male | Male | Female | Female | Female | Female |
| Living situation | Living alone | Living together | Living together | Living alone | Living alone | Living alone |
| Aetiology of HF | Hypertension arrhythmia | Hypertension arrhythmia | Ischaemic disease | Hypertension | Persistent foramen ovale | Ischaemic disease |
| NYHA class, III | III | III | II | III | III | II |
| NT-proBNP (pg/mL) baseline | 4922 | 893 | 399 | 877 | 5390 | 2951 |
| Types of HF | HFmrEF | — | HFpEF | HFmrEF | HFmrEF | HFpEF |
| Body mass index | 27.6 | 37.0 | — | 27.3 | — | 28.2 |
| Height, cm | 170 | 185 | — | 148 | — | 161 |
| Weight, kg | 79.7 | 126.5 | 65.6 | 59.8 | 98.8 | 73 |
| Chronic obstructive pulmonary disease /asthma | No | Yes | No | Yes | Yes | Yes |
| Diabetes | Yes, type 2 | Yes, type 1 | No | No | No | Yes, type 2 |
| Renal failure | Yes | No | No | No | Yes | No |
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . |
|---|---|---|---|---|---|---|
| Age | 75 | 78 | 87 | 86 | 88 | 93 |
| Gender | Male | Male | Female | Female | Female | Female |
| Living situation | Living alone | Living together | Living together | Living alone | Living alone | Living alone |
| Aetiology of HF | Hypertension arrhythmia | Hypertension arrhythmia | Ischaemic disease | Hypertension | Persistent foramen ovale | Ischaemic disease |
| NYHA class, III | III | III | II | III | III | II |
| NT-proBNP (pg/mL) baseline | 4922 | 893 | 399 | 877 | 5390 | 2951 |
| Types of HF | HFmrEF | — | HFpEF | HFmrEF | HFmrEF | HFpEF |
| Body mass index | 27.6 | 37.0 | — | 27.3 | — | 28.2 |
| Height, cm | 170 | 185 | — | 148 | — | 161 |
| Weight, kg | 79.7 | 126.5 | 65.6 | 59.8 | 98.8 | 73 |
| Chronic obstructive pulmonary disease /asthma | No | Yes | No | Yes | Yes | Yes |
| Diabetes | Yes, type 2 | Yes, type 1 | No | No | No | Yes, type 2 |
| Renal failure | Yes | No | No | No | Yes | No |
HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; NYHA, New York Heart Association.
| . | ReDS . | V-scan . | NYHA . | NT-proBNP (pg/mL) . | Symptom (range 0–10) . | Time to obtain measurements (min) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Pleural effusion . | B-lines . | IVC > 21 mm . | A collapsibility index < 50% . | Dyspnoea . | Oedema . | Fatigue . | ReDS . | V-scan . | ||
| Patient 1 | ||||||||||||
| 2023/1/11 | 45 | — | — | + | + | III | 4922 | — | — | — | 10 | 10 |
| 2023/3/1 | 47 | — | — | + | + | III | 4226 | 5 | 5 | 7 | 10 | 10 |
| Patient 2 | ||||||||||||
| 2023/3/1 | 31 | — | — | — | — | III | 1132 | 7 | 6 | 7 | 10 | 10 |
| 2023/5/4 | 30 | — | — | — | — | — | 1299 | 5 | 6 | 4 | 5 | 7 |
| Patient 3 | ||||||||||||
| 2023/1/18 | 31 | — | — | — | — | II | 399 | 3 | 2 | 2 | 6 | 8 |
| 2023/3/7 | 25 | — | — | — | — | II | 344 | 2 | 2 | 1 | 5 | 7 |
| 2023/4/19 | 36 | — | + | — | — | II | 373 | 5 | 5 | 3 | 4 | 5 |
| Patient 4 | ||||||||||||
| 2023/1/18 | 25 | — | + | — | — | III | 877 | 5 | 0 | 9 | 5 | 5 |
| 2023/2/14 | 23 | — | — | — | — | III | 1680 | 5 | 0 | 10 | 5 | 5 |
| 2023/3/28 | 22 | — | — | — | — | III | 1308 | 0 | 0 | 5 | 3 | 4 |
| Patient 5 | ||||||||||||
| 2023/2/1 | 33 | — | — | Unable to find IVC | III | 5390 | — | — | — | 6 | 5 | |
| 2023/3/1 | 38 | — | — | Unable to find IVC | III | 5381 | — | — | — | 5 | 5 | |
| 2023/4/4 | 38 | — | — | Unable to find IVC | III | 5240 | 5 | 8 | 5 | 4.5 | 4 | |
| Patient 6 | ||||||||||||
| 2023/5/4 | 41 | — | + | — | — | II | 2951 | 5 | 0 | 8 | 5 | 10 |
| 2023/6/9 | 31 | — | — | Unable to find IVC | II | 2630 | 5 | 0 | 5 | 5 | 10 | |
| . | ReDS . | V-scan . | NYHA . | NT-proBNP (pg/mL) . | Symptom (range 0–10) . | Time to obtain measurements (min) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Pleural effusion . | B-lines . | IVC > 21 mm . | A collapsibility index < 50% . | Dyspnoea . | Oedema . | Fatigue . | ReDS . | V-scan . | ||
| Patient 1 | ||||||||||||
| 2023/1/11 | 45 | — | — | + | + | III | 4922 | — | — | — | 10 | 10 |
| 2023/3/1 | 47 | — | — | + | + | III | 4226 | 5 | 5 | 7 | 10 | 10 |
| Patient 2 | ||||||||||||
| 2023/3/1 | 31 | — | — | — | — | III | 1132 | 7 | 6 | 7 | 10 | 10 |
| 2023/5/4 | 30 | — | — | — | — | — | 1299 | 5 | 6 | 4 | 5 | 7 |
| Patient 3 | ||||||||||||
| 2023/1/18 | 31 | — | — | — | — | II | 399 | 3 | 2 | 2 | 6 | 8 |
| 2023/3/7 | 25 | — | — | — | — | II | 344 | 2 | 2 | 1 | 5 | 7 |
| 2023/4/19 | 36 | — | + | — | — | II | 373 | 5 | 5 | 3 | 4 | 5 |
| Patient 4 | ||||||||||||
| 2023/1/18 | 25 | — | + | — | — | III | 877 | 5 | 0 | 9 | 5 | 5 |
| 2023/2/14 | 23 | — | — | — | — | III | 1680 | 5 | 0 | 10 | 5 | 5 |
| 2023/3/28 | 22 | — | — | — | — | III | 1308 | 0 | 0 | 5 | 3 | 4 |
| Patient 5 | ||||||||||||
| 2023/2/1 | 33 | — | — | Unable to find IVC | III | 5390 | — | — | — | 6 | 5 | |
| 2023/3/1 | 38 | — | — | Unable to find IVC | III | 5381 | — | — | — | 5 | 5 | |
| 2023/4/4 | 38 | — | — | Unable to find IVC | III | 5240 | 5 | 8 | 5 | 4.5 | 4 | |
| Patient 6 | ||||||||||||
| 2023/5/4 | 41 | — | + | — | — | II | 2951 | 5 | 0 | 8 | 5 | 10 |
| 2023/6/9 | 31 | — | — | Unable to find IVC | II | 2630 | 5 | 0 | 5 | 5 | 10 | |
Heart failure symptoms including dyspnoea, oedema, and fatigue was assessed by a visual analogue scale ranging from 0 to 10, with higher score indicating more symptoms.
IVC, inferior vena cava; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; ReDS, remote dielectric sensing technology.
| . | ReDS . | V-scan . | NYHA . | NT-proBNP (pg/mL) . | Symptom (range 0–10) . | Time to obtain measurements (min) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Pleural effusion . | B-lines . | IVC > 21 mm . | A collapsibility index < 50% . | Dyspnoea . | Oedema . | Fatigue . | ReDS . | V-scan . | ||
| Patient 1 | ||||||||||||
| 2023/1/11 | 45 | — | — | + | + | III | 4922 | — | — | — | 10 | 10 |
| 2023/3/1 | 47 | — | — | + | + | III | 4226 | 5 | 5 | 7 | 10 | 10 |
| Patient 2 | ||||||||||||
| 2023/3/1 | 31 | — | — | — | — | III | 1132 | 7 | 6 | 7 | 10 | 10 |
| 2023/5/4 | 30 | — | — | — | — | — | 1299 | 5 | 6 | 4 | 5 | 7 |
| Patient 3 | ||||||||||||
| 2023/1/18 | 31 | — | — | — | — | II | 399 | 3 | 2 | 2 | 6 | 8 |
| 2023/3/7 | 25 | — | — | — | — | II | 344 | 2 | 2 | 1 | 5 | 7 |
| 2023/4/19 | 36 | — | + | — | — | II | 373 | 5 | 5 | 3 | 4 | 5 |
| Patient 4 | ||||||||||||
| 2023/1/18 | 25 | — | + | — | — | III | 877 | 5 | 0 | 9 | 5 | 5 |
| 2023/2/14 | 23 | — | — | — | — | III | 1680 | 5 | 0 | 10 | 5 | 5 |
| 2023/3/28 | 22 | — | — | — | — | III | 1308 | 0 | 0 | 5 | 3 | 4 |
| Patient 5 | ||||||||||||
| 2023/2/1 | 33 | — | — | Unable to find IVC | III | 5390 | — | — | — | 6 | 5 | |
| 2023/3/1 | 38 | — | — | Unable to find IVC | III | 5381 | — | — | — | 5 | 5 | |
| 2023/4/4 | 38 | — | — | Unable to find IVC | III | 5240 | 5 | 8 | 5 | 4.5 | 4 | |
| Patient 6 | ||||||||||||
| 2023/5/4 | 41 | — | + | — | — | II | 2951 | 5 | 0 | 8 | 5 | 10 |
| 2023/6/9 | 31 | — | — | Unable to find IVC | II | 2630 | 5 | 0 | 5 | 5 | 10 | |
| . | ReDS . | V-scan . | NYHA . | NT-proBNP (pg/mL) . | Symptom (range 0–10) . | Time to obtain measurements (min) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Pleural effusion . | B-lines . | IVC > 21 mm . | A collapsibility index < 50% . | Dyspnoea . | Oedema . | Fatigue . | ReDS . | V-scan . | ||
| Patient 1 | ||||||||||||
| 2023/1/11 | 45 | — | — | + | + | III | 4922 | — | — | — | 10 | 10 |
| 2023/3/1 | 47 | — | — | + | + | III | 4226 | 5 | 5 | 7 | 10 | 10 |
| Patient 2 | ||||||||||||
| 2023/3/1 | 31 | — | — | — | — | III | 1132 | 7 | 6 | 7 | 10 | 10 |
| 2023/5/4 | 30 | — | — | — | — | — | 1299 | 5 | 6 | 4 | 5 | 7 |
| Patient 3 | ||||||||||||
| 2023/1/18 | 31 | — | — | — | — | II | 399 | 3 | 2 | 2 | 6 | 8 |
| 2023/3/7 | 25 | — | — | — | — | II | 344 | 2 | 2 | 1 | 5 | 7 |
| 2023/4/19 | 36 | — | + | — | — | II | 373 | 5 | 5 | 3 | 4 | 5 |
| Patient 4 | ||||||||||||
| 2023/1/18 | 25 | — | + | — | — | III | 877 | 5 | 0 | 9 | 5 | 5 |
| 2023/2/14 | 23 | — | — | — | — | III | 1680 | 5 | 0 | 10 | 5 | 5 |
| 2023/3/28 | 22 | — | — | — | — | III | 1308 | 0 | 0 | 5 | 3 | 4 |
| Patient 5 | ||||||||||||
| 2023/2/1 | 33 | — | — | Unable to find IVC | III | 5390 | — | — | — | 6 | 5 | |
| 2023/3/1 | 38 | — | — | Unable to find IVC | III | 5381 | — | — | — | 5 | 5 | |
| 2023/4/4 | 38 | — | — | Unable to find IVC | III | 5240 | 5 | 8 | 5 | 4.5 | 4 | |
| Patient 6 | ||||||||||||
| 2023/5/4 | 41 | — | + | — | — | II | 2951 | 5 | 0 | 8 | 5 | 10 |
| 2023/6/9 | 31 | — | — | Unable to find IVC | II | 2630 | 5 | 0 | 5 | 5 | 10 | |
Heart failure symptoms including dyspnoea, oedema, and fatigue was assessed by a visual analogue scale ranging from 0 to 10, with higher score indicating more symptoms.
IVC, inferior vena cava; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; ReDS, remote dielectric sensing technology.
Patient 2
A 78-year-old man characterized by HF (NYHA III), obesity, and chronic obstructive pulmonary disease reported dyspnoea at the first follow-up visit. Despite his complaints, the primary care nurse was unable to detect the presence of B-lines and recorded a low ReDS value of 31. Consequently, the nurse attributed the patient’s dyspnoea to chronic obstructive pulmonary disease rather than HF. The nurse felt that both devices proved valuable in distinguishing between symptoms associated with chronic obstructive pulmonary disease and HF exacerbations, facilitating her decision-making process regarding medication optimization for a patient facing multiple comorbidities. However, given the patient’s muscular physique, uncertainty regarding the accuracy of the obtained ReDS values arose during follow-up visits. From this case, we learned that ReDS and V-scan are helpful in distinguishing whether dyspnoea is due to HF or chronic obstructive pulmonary disease. However, the patient’s body shape contributed to uncertainty with ReDS measurements.
Patient 3
An 87-year-old female diagnosed with HF (NYHA II) consistently exhibited normal ReDS values (31 and 30, respectively), and V-scan did not detect any B-lines nor pleural effusion, and IVC was normal during the first and second visits. Consequently, the nurse concluded that the patient’s HF was stable. At the third visit, the patient presented exacerbated dyspnoea, oedema, and fatigue. Upon examination, the nurse observed a notable increase in ReDS values from 25 to 36, accompanied by a significant presence of B-lines. The nurse confirmed HF deterioration. Throughout the process of measuring both ReDS and V-scan, the primary care nurse did not encounter any technical or practical difficulties. Due to the patient’s petite stature, fitting the ReDS vest was easily accomplished. Therefore, this case demonstrates that both ReDS and V-scan assist in recognizing both stable HF and HF deterioration, and these measurements are easier to take on patients with petite stature.
Patient 4
An 86-year-old female presented with HF. During the initial visits, her ReDS value was normal at 25, the N-terminal pro-B-type natriuretic peptide (NT-proBNP) values were also normal for her (877 pg/mL). However, B-lines were detected by the primary care nurse. The nurse assessed that the patient had a stable HF regardless of the V-scan images; therefore, she opted not to alter their medication. At the second visits, her ReDS values indicated optimal fluid status, and V-scan did not reveal any signs of pulmonary congestion. Consequently, the primary care nurse concluded that the patient’s HF was stable and decided to resume optimizing HF medication initiating enalapril. During the third visit, the nurse confirmed the stable HF status through ReDS values and V-scan results. The primary care nurse expressed confidence in the ReDS value, and it only took 3 min to obtain ReDS measurements during the third visit. From this case, we learned that the nurse has developed confidence in ReDS measurement, and both devices help assess stable HF and optimize HF medication. Meanwhile, the case also highlights the possibility of misinterpreting images from V-scan.
Patient 5
An 88-year-old female had HF with preserved ejection fraction (NYHA III), asthma, and chronic kidney disease. Her recent NT-proBNP value was 5390 pg/mL. During her initial visit, the ReDS values were recorded at 33, indicating optimal volume status. The nurse did not detect B-lines nor pleural effusion through V-scan, and the nurse had difficulties locating the IVC. In result, the nurse determined the patient’s HF condition to be stable and opted to maintain the current treatment. During the second visit, the ReDS values escalated from 33 to 38, suggesting potential hypervolemic status. The patient exhibited leg oedema, and her weight had slightly increased from 98.8 to 100.9 kg. The NT-proBNP level was continuously high at 5381. The nurse did not observe pleural effusion nor B-lines, and IVC could not be located. Based on these obtained findings, the nurse concluded that the patient was experiencing right HF and cautiously increased diuretic doses. One month later at the third visit, the patient presented with dyspnoea. Consistent with the second visit, the ReDS values were slightly elevated (38%), indicating hypervolemic status, but V-scan did not detect plural effusion, any B-lines, or enlarged IVC. The nurse had uncertainties about the accuracy of the ReDS values, because she encountered challenges fitting the ReDS vest due to the patient’s abnormal spine configuration. Consequently, she trusted the findings of V-scan and concluded that the patient’s dyspnoea was most likely attributable to asthma, rather than excessive fluid in the lungs, and thought V-scan was valuable in guiding therapeutic decisions regarding diuretic administration. In this case, we learned that both ReDS and V-scan help monitor changes in lung congestion. Although the ReDS values slightly increased, the nurse distrusted the ReDS and instead placed her confidence in the V-scan.
Patient 6
A 93-year-old female presented with HF with preserved ejection fraction (NYHA II), chronic obstructive pulmonary disease, and diabetes. During her initial visit, she experienced worsening dyspnoea and fatigue. The ReDS value was high at 41, indicating hypervolemic status, which was confirmed by B-lines in the V-scan. The primary care nurse noted the deterioration of HF and initiated treatment with an SGLT2 inhibitor, empagliflozin. However, she did not change diuretics dosages. Subsequently, when the patient visited a cancer clinic at the university hospital, her furosemide dose was increased by other healthcare professionals. One month later, upon returning to the primary care centre, the nurse observed a decrease in ReDS values from 41 to 31, accompanied by the absence of B-lines on the V-scan. The patient reported reduced dyspnoea, and both NT-proBNP levels and weight showed slight reductions. Consequently, the nurse assessed that the patient’s HF status had improved and decided to continue with the current medication regimen. Additionally, the nurse reflected that the dose of diuretics should have been increased earlier based on the obtained findings. During both visits, the nurse reported difficulties interpreting the obtained images and identifying the location of the IVC. This case emphasizes the time required for primary care nurses to acquire the necessary skills to use V-scan effectively. It also underscores the necessity for an algorithm based on ReDS values and suggests that ReDS is valuable for monitoring responses to diuretics.
Discussion
These experiences of a primary care nurse describe several pros and cons of using the ReDS and V-scan as tools to assess fluid status in patients with HF and comorbidities in primary care settings. In terms of pros, both ReDS and V-scan proved valuable aids for the primary care nurse in assessing the lung congestion of HF patients. They facilitated the determination of whether the patient’s condition had worsened, remained stable, or improved over time. These devices also might assist in verifying and confirming physical examination findings, including lung auscultation and subjective symptoms reported by the patient, such as dyspnoea and leg oedema. Additionally, they seemed to contribute to decisions on medication optimization, primarily through adjustments to diuretic dosages and observation of treatment responses. Furthermore, these devices provide valuable support in distinguishing symptoms of asthma or chronic obstructive pulmonary disease from HF worsening.
On the other hand, several challenges and practical issues with both ReDS and V-scan were identified. The nurse had difficulty using the ReDS vest on patients who had hunched backs or a high body mass index. Her decision to trust the V-scan was not based on clinical expertise or data, but rather on her lack of trust in the ReDS. Additionally, there were instances where the nurse initially misunderstood the value of ReDS and failed to adjust diuretics, even when ReDS indicated hypervolemic status, leading to subsequent regret. This underscores the importance of healthcare professionals understanding both the technology and the underlying pathophysiology and the support available. Given the potential of ReDS to detect early signs of physiological changes in the progression of worsening HF, the ReDS values can increase without any other HF symptoms. These findings highlight the necessity for algorithms to accurately interpret ReDS values and provide management plans, including adjustments to diuretic doses.2 Regarding V-scan, although the primary care nurse appreciated its ability to visualize pulmonary congestion, the nurse often struggled with generating and interpreting ultrasound images, despite having trained with a HF nurse on the V-scan in a primary care centre. Continuous training may be required to effectively utilize V-scan in clinical practice. Previous studies have indicated that cardiac nurses could effectively use portable handheld ultrasound devices in hospital-based outpatient settings.11,15,16 Therefore, this inconsistency might be partially attributed to the differences in the environment—primary care vs. hospital settings—where support from cardiac and ultrasound specialists is easily accessible, as are differences in cardiac care background knowledge. We acknowledge that our discussion is based on the insights of a primary care nurse with experience in inpatient cardiology. To effectively adopt new technologies, it is crucial to consider the experiences and perspectives of various healthcare professionals, including HF specialist nurses and physicians.
In conclusion, both ReDS and V-scan can function as valuable decision support tools for primary care nurses in assessing HF status and optimizing HF medication. Nevertheless, our experience revealed several challenges inherent in their use. These challenges may include technical difficulties, interpretational complexities, or limitations among specific patient populations. Acknowledging and addressing these challenges will be crucial for maximizing the utility of these devices in primary care settings. Given that ReDS may not necessitate specialized cardiac knowledge, it might be more suitable for integration into primary care settings. However, the development of an algorithm to interpret ReDS values and generate comprehensive management plans is imperative for its appropriate and effective utilization.
Our paper, which describes a nurse’s experiences and discusses the pros and cons of adopting new technologies as well as potential strategies to address these challenges, represents an important step towards their effective implementation in various environments, including among diverse healthcare professionals and in different clinical settings.
Author contributions
Naoko P. Kato, RN, PhD (Conceptualization [equal], Data curation [supporting], Formal analysis [supporting], Funding acquisition [lead], Investigation [supporting], Methodology [equal], Project administration [equal], Writing—original draft [equal], Writing—review & editing [equal]); Marie Mattisson, RN, PhD (Data curation [lead], Formal analysis [lead], Project administration [supporting], Writing—original draft [equal], Writing—review & editing [supporting]); Pernilla Grahn, RN (Conceptualization [supporting], Investigation [lead], Writing—review & editing [supporting]); Maria Liljeroos, RN, PhD (Conceptualization [supporting], Funding acquisition [supporting], Investigation [supporting], Supervision [equal], Writing—review & editing [equal]); Peter Johansson, RN, PhD (Conceptualization [equal], Investigation [supporting], Methodology [supporting], Supervision [supporting], Writing—review & editing [equal]); Anna Strömberg, RN, PhD (Conceptualization [equal], Formal analysis [supporting], Funding acquisition [supporting], Investigation [supporting], Methodology [supporting], Project administration [supporting], Supervision [supporting], Writing—review & editing [equal]); and Tiny Jaarsma, RN, PhD (Conceptualization [equal], Data curation [supporting], Formal analysis [supporting], Funding acquisition [lead], Methodology [supporting], Project administration [supporting], Supervision [equal], Writing—original draft [equal], Writing—review & editing [supporting])
Funding
This work was supported by the Promobilia Foundation (to T.J.), the Medical Research Council of Southeast Sweden (FORSS) (753301 to T.J.), the Kamprad Family Foundation (20210053 to N.P.K.), the Swedish Heart Lung Foundation (20210322 to N.P.K.), and the Japan Society for the Promotion of Science (JSPS) Grant-in- Aid for Scientific Research (KAKENHI) (18K17517 to N.P.K.).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Author notes
Conflict of interest: none declared.




Comments