-
PDF
- Split View
-
Views
-
Cite
Cite
Kohei Nozaki, Nobuaki Hamazaki, Kentaro Kamiya, Shota Uchida, Takumi Noda, Kensuke Ueno, Kazuki Hotta, Emi Maekawa, Atsuhiko Matsunaga, Minako Yamaoka-Tojo, Junya Ako, Association between walking speed early after admission and all-cause death and/or re-admission in patients with acute decompensated heart failure, European Journal of Cardiovascular Nursing, Volume 23, Issue 4, May 2024, Pages 374–381, https://doi.org/10.1093/eurjcn/zvad092
Close - Share Icon Share
Abstract
Patients with heart failure (HF) frequently experience decreased physical function, including walking speed. Slower walking speed is associated with poorer prognosis. However, most of these reports focused on patients with stable HF, and the relationship between walking speed in acute phase and clinical outcomes is unclear. Therefore, we aimed to investigate the associations between walking speed early after admission and clinical events in patients with acute decompensated HF (ADHF).
We reviewed consecutive 1391 patients admitted due to ADHF. We measured walking speed the first time to walk on the ward more than 10 m after admission, and the speed within 4 days after admission was included in this study. The primary outcome was combined events (all-cause death and/or re-admission due to HF). The follow-up period was up to 1 year from the discharge. The study population had a median age of 74 years [interquartile range (IQR): 65–80 years], and 35.9% of patients were females. The median walking speed was 0.70 m/s (IQR: 0.54–0.88 m/s). Combined events occurred in 429 (30.8%) patients. Faster walking speed was independently associated with lower rate of combined events (adjusted hazard ratio per 0.1 m/s increasing: 0.951, 95% confidence interval: 0.912–0.992).
Faster walking speed within 4 days after admission was associated with favourable clinical outcomes in patients with ADHF. The results suggest that measuring walking speed in acute phase is useful for earlier risk stratification.
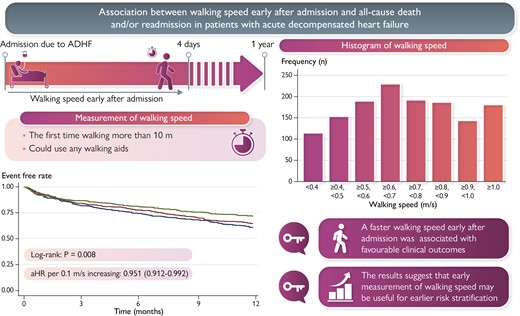
Walking speed within 4 days after admission predicts clinical outcomes in patients with acute decompensated heart failure (ADHF).
Walking speed in acute phase predicts clinical outcomes across various subgroups, particularly in older patients.
Measurement of walking speed early after admission may help in earlier risk stratification in patients with ADHF.
Introduction
The increasing prevalence of heart failure (HF) is a major public health concern.1,2 Patients with HF often experience impaired physical function such as physical frailty, and these patients are more likely to have poor outcomes.3,4 In particular, decreased walking speed, a critical component of frailty,5,6 has been identified as a risk marker for higher incidence of re-hospitalization and all-cause death in patients with HF.7,8 However, most studies have measured walking speed in the stable phase, and few reports have examined the association between walking speed measured early after admission and clinical outcomes in patients with acute decompensated HF (ADHF). As recent research suggests that the early-phase rehabilitation promote better recovery of physical function and more favourable clinical outcomes in patients with ADHF,9–11 walking speed measured in the acute phase could be a valuable marker for early risk stratification to predict clinical outcomes. Early risk stratification including prognosis after admission due to ADHF could help facilitate appropriate clinical management and therapeutic decision-making. Since the measurement of walking speed is a safe, easy, and immediately measurable method, it may be of clinical value if walking speed in the early post-hospitalization period predicts outcomes. Therefore, we aimed to investigate the association between walking speed early after admission and all-cause death and/or re-admission in patients with ADHF.
Methods
Study population
This is the cohort study, and we studied 3052 consecutive patients who admitted to our hospital due to ADHF between January 2011 and December 2021. Exclusion criteria were as follows: need for advanced mechanical support, including intubation, intra-aortic balloon pumping, catheter-mounted left ventricular assist device, and extracorporeal membrane oxygenation; inability to walk more than 10 m independently before admission; could not start walking within 4 days after admission; and missing walking speed measurement. Acute decompensated heart failure was defined according to the Framingham criteria (see Supplementary material online, Table S1).12
This study was conducted in accordance with the tenets of the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of our institution (KMEO B18-075). Owing to the fact that all outcome measures were collected as a part of routine care, we made the information on the research public by opt-out,13 and informed consent was waived.
Data collection
Data were collected from patients’ electronic medical records. Clinical data including biochemical and echocardiographic data were measured on admission to our hospital. Estimated glomerular filtration rate (eGFR) was calculated using the formula recommended by the Japanese Society of Nephrology: 194 × (serum creatinine)1.094 × (age)0.287 for males and 194 × (serum creatinine)1.094 × (age)0.287 × 0.739 for females.14 Left ventricular ejection fraction (LVEF) was estimated using Simpson’s method on two-dimensional echocardiograms. Pre-admission physical activity levels were assessed using the three-question physical activity assessment tools by interview.15 The instruments measured the number of vigorous-intensity activity lasting 20 min in duration and walking or moderate-intensity activity that were 30 min in a usual week. According to the scores, the amount of total activity was classified as minimal, low, adequate, and high.
Walking speed measurement in the early phase after admission
We measured walking speed once at the first ambulation on the ward more than 10 m after admission, and the speed measured within 4 days after admission was included in this study, because the standard programme published by the Japanese Society of Cardiac Rehabilitation describes starting rehabilitation from a sitting position,16,17 and walking is expected to start somewhat later. Initial ambulation was initiated after the symptoms of HF had improved and after discussion with physicians, nurses, and physical therapists familiar with cardiac rehabilitation to determine if ambulation was feasible. To measure walking speed, the patients were asked to walk at their usual speed and were measured for the time taken to walk in the 10 m walkway. Patients were allowed to use any walking aids, including canes and walkers.
Outcomes
The primary outcome of this study was a composite endpoint of re-hospitalization due to HF and all-cause death, and the secondary outcome was all-cause death. Time to the endpoint was calculated as the number of days up to 1 year from the date of walking speed measurement to the date of the event.
Statistical analyses
Representation of data and comparison of patient background factors
The variables are presented as the median (interquartile range). Categorical variables are expressed as numbers and percentages. The patients were divided into three groups according to the tertile of the walking speed: the fast group; the moderate group; and the slow group. As there is no cut-off value for walking speed in the acute phase, we chose the tertile for categorizing the groups. Baseline patient characteristics were compared using the Kruskal–Wallis test for continuous variables and the χ2 test for categorical variables.
Survival analyses
Event-free survival curves were constructed using the Kaplan–Meier survival method and were compared with log-rank test. For the outcome of combined events, multivariable Cox regression analyses were performed for walking speed adjusted for age; sex; body mass index; New York Heart Association class III/IV; systolic blood pressure; LVEF; current smoking status; history of HF (whether or not a history of hospitalization); hypertension, diabetes, coronary artery disease, chronic obstructive pulmonary disease, atrial fibrillation, and stroke; eGFR; haemoglobin; serum sodium level; serum albumin; log-transformed BNP; prescription of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, beta-blocker, and mineralocorticoid receptor antagonist at admission; and physical activity level before admission. We selected these variables according to their clinical importance and based on previous studies.18
Sensitivity analyses
We also performed a sensitivity analysis to confirm the robustness of the results of this study. The definition of walking speed in the early phase after admission was re-defined as measured within 5 days of admission, and the association between gait speed measured during that period and outcomes was examined, adjusted for the same confounders.
Subgroup analyses
To investigate the potential effect modification on the association of the walking speed with combined events, we performed subgroup analyses in various subgroups relevant to HF prognosis, including the sex, age (stratified at 65 years), body mass index (BMI) (stratified at 25 kg/m2), LVEF (stratified at 40% and 50%), and history of prior HF admission with adjustments for the same confounders. A P-value of <0.1 was defined as a statistical interaction.
Handle for missing data
Multiple imputation was used to account for the missing covariate data to construct multivariable Cox regression models. We created 20 data sets using a chained equations procedure.19 Parameter estimates were obtained for each data set and subsequently combined to produce an integrated result using the method described by Barnard and Rubin.20
Analyses were performed using SPSS version 27.0 (IBM Corporation, Armonk, NY, USA) and Stata version 15.0 (StataCorp LP, College Station, TX, USA). In all analyses, a two-tailed P < 0.05 was taken to indicate statistical significance.20
Results
Study population
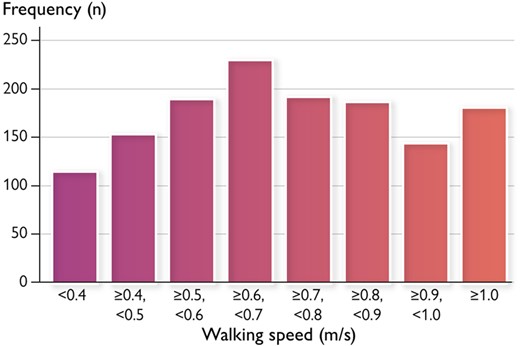
Among the potential number of 3052 patients, we analysed 1391 patients after excluding those who required advanced mechanical support (n = 492), those who were unable to walk before admission (n = 470), those who could not start ambulation within 4 days after admission (n = 528), and missing walking speed measurement (n = 171). Patients’ characteristics at admission are shown in Table 1. The median age was 74 years old, and 35.9% of the patients were females. The median value of LVEF, BNP, and walking speed were 40%, 798 pg/mL, and 0.70 m/s, respectively. A histogram of walking speed is shown in Figure 1. The most frequent distribution was for walking speeds ≥0.6 m/s and <0.7 m/s. Patients with slower walking speeds were significantly older, were more female, were more severely symptomatic, had faster heart rates, were more likely to have a history of stroke, were fewer smokers, and had lower haemoglobin, albumin, and eGFR and lower total physical activities before admission than those with faster walking speed.
| . | Missing data . | Overall (N = 1391) . | Slow (N = 464) . | Middle (N = 464) . | Fast (N = 463) . | P value . |
|---|---|---|---|---|---|---|
| Age, years | 0 | 74 (65, 80) | 78 (70, 84) | 73 (64, 80) | 71 (61, 77) | <0.001 |
| Female, n (%) | 0 | 499 (35.9) | 231 (49.8) | 158 (34.1) | 110 (23.8) | <0.001 |
| Body mass index, kg/m2 | 0 | 23.3 (20.8, 26.4) | 23.1 (20.5, 26.5) | 23.3 (21.0, 26.8) | 23.4 (21.2, 26.2) | |
| NYHA functional class, n (%) | ||||||
| III/IV | 114 | 1226 (88.1) | 413 (89.0) | 413 (89.0) | 386 (83.4) | <0.001 |
| Systolic blood pressure, mmHg | 0 | 128 (108, 150) | 129 (107, 154) | 128 (108, 150) | 128 (109, 150) | 0.857 |
| Heart rate, b.p.m. | 10 | 82 (70, 102) | 84 (70, 106) | 84 (70, 104) | 80 (70, 97) | 0.007 |
| LVEF, % | 90 | 40 (27, 56) | 40 (26, 56) | 38 (26, 55) | 40 (29, 56) | 0.250 |
| Comorbidities, n (%) | ||||||
| Hypertension | 0 | 967 (69.5) | 335 (72.2) | 319 (68.8) | 313 (67.6) | 0.286 |
| Diabetes mellitus | 0 | 622 (44.7) | 213 (45.9) | 207 (44.6) | 202 (43.6) | 0.783 |
| Dyslipidaemia | 0 | 554 (39.8) | 182 (39.2) | 185 (39.9) | 187 (40.4) | 0.936 |
| Atrial fibrillation | 0 | 619 (44.5) | 200 (43.1) | 215 (46.3) | 204 (44.1) | 0.596 |
| Ischaemic aetiology | 0 | 413 (29.7) | 135 (29.1) | 134 (28.9) | 144 (31.1) | 0.717 |
| COPD | 0 | 67 (4.8) | 19 (4.1) | 29 (6.3) | 19 (4.1) | 0.210 |
| Stroke | 0 | 167 (12.0) | 82 (17.7) | 50 (10.8) | 35 (7.6) | 0.002 |
| Prior HF admission, n (%) | 0 | 629 (45.2) | 221 (47.6) | 211 (45.5) | 216 (46.7) | 0.805 |
| Current smoker, n (%) | 19 | 215 (15.5) | 53 (11.4) | 79 (17.0) | 83 (17.9) | 0.012 |
| Laboratory data | ||||||
| Haemoglobin, g/dL | 2 | 12.1 (10.4, 13.8) | 11.6 (10.0, 13.3) | 12.1 (10.0, 13.9) | 12.1 (10.6, 14.3) | <0.001 |
| Albumin, g/dL | 15 | 3.4 (3.1, 3.7) | 3.3 (3.0, 3.6) | 3.4 (3.1, 3.7) | 3.5 (3.1, 3.8) | <0.001 |
| Creatinine, mg/dL | 2 | 1.22 (0.94, 1.74) | 1.22 (1.01, 1.90) | 1.24 (0.96, 1.78) | 1.18 (0.89, 1.65) | 0.086 |
| eGFR, mL/min/1.73 m2 | 2 | 43 (28, 57) | 40 (27, 53) | 41 (28, 54) | 47 (31, 63) | <0.001 |
| Sodium, mEq/mL | 2 | 138 (136, 140) | 138 (135, 141) | 138 (136, 140) | 139 (136, 140) | 0.162 |
| BNP, pg/mL | 144 | 798 (421, 1443) | 876 (477, 1570) | 845 (454, 1487) | 644 (354, 1185) | <0.001 |
| Medications, n (%) | ||||||
| ACEI/ARB | 0 | 820 (58.9) | 274 (59.0) | 275 (59.3) | 271 (58.5) | 0.944 |
| β-blocker | 0 | 701 (50.4) | 247 (53.2) | 226 (48.7) | 228 (49.2) | 0.558 |
| MRA | 0 | 640 (46.0) | 195 (42.0) | 223 (48.1) | 222 (47.9) | 0.091 |
| Physical activity before admission | 331 | <0.001 | ||||
| Minimum | 654 (47.0) | 259 (55.8) | 216 (46.6) | 179 (38.7) | ||
| Low | 126 (9.1) | 34 (7.3) | 47 (10.1) | 45 (9.7) | ||
| Adequate | 280 (20.1) | 52 (11.2) | 107 (23.1) | 121 (26.1) | ||
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Date of walking speed measurement | 0 | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 0.101 |
| Walking speed, m/s | 0 | 0.70 (0.54, 0.88) | 0.48 (0.40, 0.54) | 0.70 (0.66, 0.76) | 0.96 (0.88, 1.06) | <0.001 |
| . | Missing data . | Overall (N = 1391) . | Slow (N = 464) . | Middle (N = 464) . | Fast (N = 463) . | P value . |
|---|---|---|---|---|---|---|
| Age, years | 0 | 74 (65, 80) | 78 (70, 84) | 73 (64, 80) | 71 (61, 77) | <0.001 |
| Female, n (%) | 0 | 499 (35.9) | 231 (49.8) | 158 (34.1) | 110 (23.8) | <0.001 |
| Body mass index, kg/m2 | 0 | 23.3 (20.8, 26.4) | 23.1 (20.5, 26.5) | 23.3 (21.0, 26.8) | 23.4 (21.2, 26.2) | |
| NYHA functional class, n (%) | ||||||
| III/IV | 114 | 1226 (88.1) | 413 (89.0) | 413 (89.0) | 386 (83.4) | <0.001 |
| Systolic blood pressure, mmHg | 0 | 128 (108, 150) | 129 (107, 154) | 128 (108, 150) | 128 (109, 150) | 0.857 |
| Heart rate, b.p.m. | 10 | 82 (70, 102) | 84 (70, 106) | 84 (70, 104) | 80 (70, 97) | 0.007 |
| LVEF, % | 90 | 40 (27, 56) | 40 (26, 56) | 38 (26, 55) | 40 (29, 56) | 0.250 |
| Comorbidities, n (%) | ||||||
| Hypertension | 0 | 967 (69.5) | 335 (72.2) | 319 (68.8) | 313 (67.6) | 0.286 |
| Diabetes mellitus | 0 | 622 (44.7) | 213 (45.9) | 207 (44.6) | 202 (43.6) | 0.783 |
| Dyslipidaemia | 0 | 554 (39.8) | 182 (39.2) | 185 (39.9) | 187 (40.4) | 0.936 |
| Atrial fibrillation | 0 | 619 (44.5) | 200 (43.1) | 215 (46.3) | 204 (44.1) | 0.596 |
| Ischaemic aetiology | 0 | 413 (29.7) | 135 (29.1) | 134 (28.9) | 144 (31.1) | 0.717 |
| COPD | 0 | 67 (4.8) | 19 (4.1) | 29 (6.3) | 19 (4.1) | 0.210 |
| Stroke | 0 | 167 (12.0) | 82 (17.7) | 50 (10.8) | 35 (7.6) | 0.002 |
| Prior HF admission, n (%) | 0 | 629 (45.2) | 221 (47.6) | 211 (45.5) | 216 (46.7) | 0.805 |
| Current smoker, n (%) | 19 | 215 (15.5) | 53 (11.4) | 79 (17.0) | 83 (17.9) | 0.012 |
| Laboratory data | ||||||
| Haemoglobin, g/dL | 2 | 12.1 (10.4, 13.8) | 11.6 (10.0, 13.3) | 12.1 (10.0, 13.9) | 12.1 (10.6, 14.3) | <0.001 |
| Albumin, g/dL | 15 | 3.4 (3.1, 3.7) | 3.3 (3.0, 3.6) | 3.4 (3.1, 3.7) | 3.5 (3.1, 3.8) | <0.001 |
| Creatinine, mg/dL | 2 | 1.22 (0.94, 1.74) | 1.22 (1.01, 1.90) | 1.24 (0.96, 1.78) | 1.18 (0.89, 1.65) | 0.086 |
| eGFR, mL/min/1.73 m2 | 2 | 43 (28, 57) | 40 (27, 53) | 41 (28, 54) | 47 (31, 63) | <0.001 |
| Sodium, mEq/mL | 2 | 138 (136, 140) | 138 (135, 141) | 138 (136, 140) | 139 (136, 140) | 0.162 |
| BNP, pg/mL | 144 | 798 (421, 1443) | 876 (477, 1570) | 845 (454, 1487) | 644 (354, 1185) | <0.001 |
| Medications, n (%) | ||||||
| ACEI/ARB | 0 | 820 (58.9) | 274 (59.0) | 275 (59.3) | 271 (58.5) | 0.944 |
| β-blocker | 0 | 701 (50.4) | 247 (53.2) | 226 (48.7) | 228 (49.2) | 0.558 |
| MRA | 0 | 640 (46.0) | 195 (42.0) | 223 (48.1) | 222 (47.9) | 0.091 |
| Physical activity before admission | 331 | <0.001 | ||||
| Minimum | 654 (47.0) | 259 (55.8) | 216 (46.6) | 179 (38.7) | ||
| Low | 126 (9.1) | 34 (7.3) | 47 (10.1) | 45 (9.7) | ||
| Adequate | 280 (20.1) | 52 (11.2) | 107 (23.1) | 121 (26.1) | ||
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Date of walking speed measurement | 0 | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 0.101 |
| Walking speed, m/s | 0 | 0.70 (0.54, 0.88) | 0.48 (0.40, 0.54) | 0.70 (0.66, 0.76) | 0.96 (0.88, 1.06) | <0.001 |
Data are expressed as median (interquartile range) and number (%). Walking speed was classified according to tertile.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
| . | Missing data . | Overall (N = 1391) . | Slow (N = 464) . | Middle (N = 464) . | Fast (N = 463) . | P value . |
|---|---|---|---|---|---|---|
| Age, years | 0 | 74 (65, 80) | 78 (70, 84) | 73 (64, 80) | 71 (61, 77) | <0.001 |
| Female, n (%) | 0 | 499 (35.9) | 231 (49.8) | 158 (34.1) | 110 (23.8) | <0.001 |
| Body mass index, kg/m2 | 0 | 23.3 (20.8, 26.4) | 23.1 (20.5, 26.5) | 23.3 (21.0, 26.8) | 23.4 (21.2, 26.2) | |
| NYHA functional class, n (%) | ||||||
| III/IV | 114 | 1226 (88.1) | 413 (89.0) | 413 (89.0) | 386 (83.4) | <0.001 |
| Systolic blood pressure, mmHg | 0 | 128 (108, 150) | 129 (107, 154) | 128 (108, 150) | 128 (109, 150) | 0.857 |
| Heart rate, b.p.m. | 10 | 82 (70, 102) | 84 (70, 106) | 84 (70, 104) | 80 (70, 97) | 0.007 |
| LVEF, % | 90 | 40 (27, 56) | 40 (26, 56) | 38 (26, 55) | 40 (29, 56) | 0.250 |
| Comorbidities, n (%) | ||||||
| Hypertension | 0 | 967 (69.5) | 335 (72.2) | 319 (68.8) | 313 (67.6) | 0.286 |
| Diabetes mellitus | 0 | 622 (44.7) | 213 (45.9) | 207 (44.6) | 202 (43.6) | 0.783 |
| Dyslipidaemia | 0 | 554 (39.8) | 182 (39.2) | 185 (39.9) | 187 (40.4) | 0.936 |
| Atrial fibrillation | 0 | 619 (44.5) | 200 (43.1) | 215 (46.3) | 204 (44.1) | 0.596 |
| Ischaemic aetiology | 0 | 413 (29.7) | 135 (29.1) | 134 (28.9) | 144 (31.1) | 0.717 |
| COPD | 0 | 67 (4.8) | 19 (4.1) | 29 (6.3) | 19 (4.1) | 0.210 |
| Stroke | 0 | 167 (12.0) | 82 (17.7) | 50 (10.8) | 35 (7.6) | 0.002 |
| Prior HF admission, n (%) | 0 | 629 (45.2) | 221 (47.6) | 211 (45.5) | 216 (46.7) | 0.805 |
| Current smoker, n (%) | 19 | 215 (15.5) | 53 (11.4) | 79 (17.0) | 83 (17.9) | 0.012 |
| Laboratory data | ||||||
| Haemoglobin, g/dL | 2 | 12.1 (10.4, 13.8) | 11.6 (10.0, 13.3) | 12.1 (10.0, 13.9) | 12.1 (10.6, 14.3) | <0.001 |
| Albumin, g/dL | 15 | 3.4 (3.1, 3.7) | 3.3 (3.0, 3.6) | 3.4 (3.1, 3.7) | 3.5 (3.1, 3.8) | <0.001 |
| Creatinine, mg/dL | 2 | 1.22 (0.94, 1.74) | 1.22 (1.01, 1.90) | 1.24 (0.96, 1.78) | 1.18 (0.89, 1.65) | 0.086 |
| eGFR, mL/min/1.73 m2 | 2 | 43 (28, 57) | 40 (27, 53) | 41 (28, 54) | 47 (31, 63) | <0.001 |
| Sodium, mEq/mL | 2 | 138 (136, 140) | 138 (135, 141) | 138 (136, 140) | 139 (136, 140) | 0.162 |
| BNP, pg/mL | 144 | 798 (421, 1443) | 876 (477, 1570) | 845 (454, 1487) | 644 (354, 1185) | <0.001 |
| Medications, n (%) | ||||||
| ACEI/ARB | 0 | 820 (58.9) | 274 (59.0) | 275 (59.3) | 271 (58.5) | 0.944 |
| β-blocker | 0 | 701 (50.4) | 247 (53.2) | 226 (48.7) | 228 (49.2) | 0.558 |
| MRA | 0 | 640 (46.0) | 195 (42.0) | 223 (48.1) | 222 (47.9) | 0.091 |
| Physical activity before admission | 331 | <0.001 | ||||
| Minimum | 654 (47.0) | 259 (55.8) | 216 (46.6) | 179 (38.7) | ||
| Low | 126 (9.1) | 34 (7.3) | 47 (10.1) | 45 (9.7) | ||
| Adequate | 280 (20.1) | 52 (11.2) | 107 (23.1) | 121 (26.1) | ||
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Date of walking speed measurement | 0 | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 0.101 |
| Walking speed, m/s | 0 | 0.70 (0.54, 0.88) | 0.48 (0.40, 0.54) | 0.70 (0.66, 0.76) | 0.96 (0.88, 1.06) | <0.001 |
| . | Missing data . | Overall (N = 1391) . | Slow (N = 464) . | Middle (N = 464) . | Fast (N = 463) . | P value . |
|---|---|---|---|---|---|---|
| Age, years | 0 | 74 (65, 80) | 78 (70, 84) | 73 (64, 80) | 71 (61, 77) | <0.001 |
| Female, n (%) | 0 | 499 (35.9) | 231 (49.8) | 158 (34.1) | 110 (23.8) | <0.001 |
| Body mass index, kg/m2 | 0 | 23.3 (20.8, 26.4) | 23.1 (20.5, 26.5) | 23.3 (21.0, 26.8) | 23.4 (21.2, 26.2) | |
| NYHA functional class, n (%) | ||||||
| III/IV | 114 | 1226 (88.1) | 413 (89.0) | 413 (89.0) | 386 (83.4) | <0.001 |
| Systolic blood pressure, mmHg | 0 | 128 (108, 150) | 129 (107, 154) | 128 (108, 150) | 128 (109, 150) | 0.857 |
| Heart rate, b.p.m. | 10 | 82 (70, 102) | 84 (70, 106) | 84 (70, 104) | 80 (70, 97) | 0.007 |
| LVEF, % | 90 | 40 (27, 56) | 40 (26, 56) | 38 (26, 55) | 40 (29, 56) | 0.250 |
| Comorbidities, n (%) | ||||||
| Hypertension | 0 | 967 (69.5) | 335 (72.2) | 319 (68.8) | 313 (67.6) | 0.286 |
| Diabetes mellitus | 0 | 622 (44.7) | 213 (45.9) | 207 (44.6) | 202 (43.6) | 0.783 |
| Dyslipidaemia | 0 | 554 (39.8) | 182 (39.2) | 185 (39.9) | 187 (40.4) | 0.936 |
| Atrial fibrillation | 0 | 619 (44.5) | 200 (43.1) | 215 (46.3) | 204 (44.1) | 0.596 |
| Ischaemic aetiology | 0 | 413 (29.7) | 135 (29.1) | 134 (28.9) | 144 (31.1) | 0.717 |
| COPD | 0 | 67 (4.8) | 19 (4.1) | 29 (6.3) | 19 (4.1) | 0.210 |
| Stroke | 0 | 167 (12.0) | 82 (17.7) | 50 (10.8) | 35 (7.6) | 0.002 |
| Prior HF admission, n (%) | 0 | 629 (45.2) | 221 (47.6) | 211 (45.5) | 216 (46.7) | 0.805 |
| Current smoker, n (%) | 19 | 215 (15.5) | 53 (11.4) | 79 (17.0) | 83 (17.9) | 0.012 |
| Laboratory data | ||||||
| Haemoglobin, g/dL | 2 | 12.1 (10.4, 13.8) | 11.6 (10.0, 13.3) | 12.1 (10.0, 13.9) | 12.1 (10.6, 14.3) | <0.001 |
| Albumin, g/dL | 15 | 3.4 (3.1, 3.7) | 3.3 (3.0, 3.6) | 3.4 (3.1, 3.7) | 3.5 (3.1, 3.8) | <0.001 |
| Creatinine, mg/dL | 2 | 1.22 (0.94, 1.74) | 1.22 (1.01, 1.90) | 1.24 (0.96, 1.78) | 1.18 (0.89, 1.65) | 0.086 |
| eGFR, mL/min/1.73 m2 | 2 | 43 (28, 57) | 40 (27, 53) | 41 (28, 54) | 47 (31, 63) | <0.001 |
| Sodium, mEq/mL | 2 | 138 (136, 140) | 138 (135, 141) | 138 (136, 140) | 139 (136, 140) | 0.162 |
| BNP, pg/mL | 144 | 798 (421, 1443) | 876 (477, 1570) | 845 (454, 1487) | 644 (354, 1185) | <0.001 |
| Medications, n (%) | ||||||
| ACEI/ARB | 0 | 820 (58.9) | 274 (59.0) | 275 (59.3) | 271 (58.5) | 0.944 |
| β-blocker | 0 | 701 (50.4) | 247 (53.2) | 226 (48.7) | 228 (49.2) | 0.558 |
| MRA | 0 | 640 (46.0) | 195 (42.0) | 223 (48.1) | 222 (47.9) | 0.091 |
| Physical activity before admission | 331 | <0.001 | ||||
| Minimum | 654 (47.0) | 259 (55.8) | 216 (46.6) | 179 (38.7) | ||
| Low | 126 (9.1) | 34 (7.3) | 47 (10.1) | 45 (9.7) | ||
| Adequate | 280 (20.1) | 52 (11.2) | 107 (23.1) | 121 (26.1) | ||
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Date of walking speed measurement | 0 | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 0.101 |
| Walking speed, m/s | 0 | 0.70 (0.54, 0.88) | 0.48 (0.40, 0.54) | 0.70 (0.66, 0.76) | 0.96 (0.88, 1.06) | <0.001 |
Data are expressed as median (interquartile range) and number (%). Walking speed was classified according to tertile.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
Associations between walking speed early after admission and outcomes
A total of 1185 (85.2%) patients completed the 1-year follow-up. During the follow-up period, 429 (30.8%) combined events (all-cause death and re-admissions due to HF) and 134 (9.6%) all-cause deaths were occurred. The Kaplan–Meier survival curve followed by log-rank test is shown in Figure 2. Slower walking speed was associated with a higher rate of both combined events and all-cause death (P = 0.008 and 0.049, respectively). Table 2 shows the results of the Cox regression analysis for combined events and all-cause mortality. Even after adjusting for the confounders, faster walking speed early after admission was associated with favourable clinical outcomes. In a sensitivity analysis, re-defining the timing of early post-admission walking speed measurement as within 5 days after admission did not noticeably affect the estimated association between walking speed and combined events (adjusted hazard ratio: 0.942, 95% confidence interval: 0.903–0.988, P = 0.011). In addition, the results of the subgroup analysis are shown in Figure 3. An interaction was observed in the subgroups of age (stratified at 65 years).
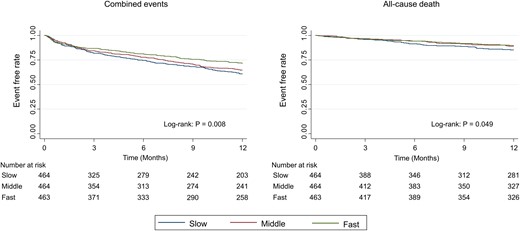
Kaplan–Meier curve for clinical events according to the tertile of walking speed.
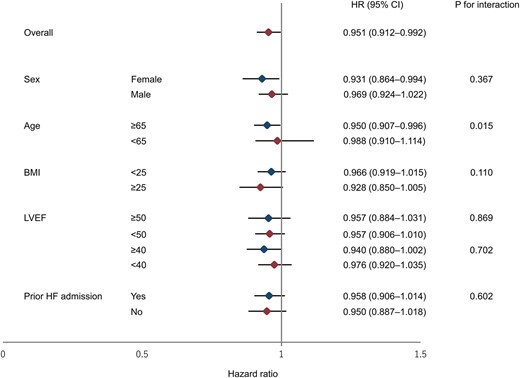
Forest plot of the hazard ratio for the association of walking speed with clinical events. BMI, body mass index; CI, confidence interval; HF, heart failure; HR, hazard ratio; and LVEF, left ventricular ejection fraction.
Results of the Cox proportional hazard models of the walking speed for outcomes
| Outcomes . | No. of events/cases . | HR (95% CI) . | P value . |
|---|---|---|---|
| Combined events (per 0.1 m/s increase) | 429/1391 (30.8%) | 0.951 (0.912–0.992) | 0.035 |
| All-cause death (per 0.1 m/s increase) | 134/1391 (9.6%) | 0.924 (0.853–0.998) | 0.048 |
| Outcomes . | No. of events/cases . | HR (95% CI) . | P value . |
|---|---|---|---|
| Combined events (per 0.1 m/s increase) | 429/1391 (30.8%) | 0.951 (0.912–0.992) | 0.035 |
| All-cause death (per 0.1 m/s increase) | 134/1391 (9.6%) | 0.924 (0.853–0.998) | 0.048 |
Adjustment variables used for combined events and all-cause death were age; sex; body mass index; New York Heart Association class III/IV; systolic blood pressure; left ventricular ejection fraction; current smoking status; history of heart failure (whether or not a history of hospitalization); hypertension, diabetes, coronary artery disease, chronic obstructive pulmonary disease, atrial fibrillation, and stroke; eGFR; haemoglobin; serum sodium level; serum albumin; log-transformed BNP; prescription of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, beta-blocker, and mineralocorticoid receptor antagonist at admission; and physical activity level before admission.
CI, confidence interval; HR, hazard ratio.
Results of the Cox proportional hazard models of the walking speed for outcomes
| Outcomes . | No. of events/cases . | HR (95% CI) . | P value . |
|---|---|---|---|
| Combined events (per 0.1 m/s increase) | 429/1391 (30.8%) | 0.951 (0.912–0.992) | 0.035 |
| All-cause death (per 0.1 m/s increase) | 134/1391 (9.6%) | 0.924 (0.853–0.998) | 0.048 |
| Outcomes . | No. of events/cases . | HR (95% CI) . | P value . |
|---|---|---|---|
| Combined events (per 0.1 m/s increase) | 429/1391 (30.8%) | 0.951 (0.912–0.992) | 0.035 |
| All-cause death (per 0.1 m/s increase) | 134/1391 (9.6%) | 0.924 (0.853–0.998) | 0.048 |
Adjustment variables used for combined events and all-cause death were age; sex; body mass index; New York Heart Association class III/IV; systolic blood pressure; left ventricular ejection fraction; current smoking status; history of heart failure (whether or not a history of hospitalization); hypertension, diabetes, coronary artery disease, chronic obstructive pulmonary disease, atrial fibrillation, and stroke; eGFR; haemoglobin; serum sodium level; serum albumin; log-transformed BNP; prescription of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, beta-blocker, and mineralocorticoid receptor antagonist at admission; and physical activity level before admission.
CI, confidence interval; HR, hazard ratio.
Discussion
Summary of the results
In the present study, we investigated whether walking speed early after hospitalization due to ADHF is associated with clinical outcomes. The main findings of our study are as follows: walking speed within 4 days after admission predicted combined events and all-cause death, after adjusting for confounders; walking speed early after admission was also useful for predicting clinical events in various subgroups. In particular, the clinical value of walking speed in the acute phase may be considerable in older patients, as an interaction for combined events was found in the subgroup of whether older or not. To our knowledge, this is the first study to demonstrate that walking speed early after admission (within 4 days) predicts combined events and all-cause death in patients admitted with ADHF. These results suggest that assessment of walking speed which can be easily and safely measured early after hospitalization is useful for earlier risk stratification to predict outcomes.
Positioning of this study in relation to previous studies
Kamiya et al.7 reported that walking speed measured at hospital discharge has prognostic capability comparable to a 6 min walking distance that is a parameter of exercise tolerance in patients with cardiovascular disease. In addition, Ozawa et al.8 reported that standardized walking speed had prognostic value for patients with HF using data from the FRAGILE-HF registry, a multicentre cohort study. Thus, walking speed is known to be a marker that predicts clinical events in patients with cardiovascular disease including HF. However, most of these reports are based on walking speeds measured in a stable phase (e.g. in patients who have not been hospitalized in the past few months or with walking speeds measured at the time of hospital discharge). Our study is the first to show that walking speed is associated with outcome in the early phase after admission, when patients are able to walk in the corridor.
Considerations on mechanisms
Although the mechanism of the association is unknown, it may be partly because walking speed is a typical index of frailty. Frailty is widely known to be associated with poorer prognosis in various populations including community-dwelling adults and patients with HF.18,21,22 Romero-Ortuno et al.23 reported that frailty assessed using the Clinical Frailty Scale prior to admission was associated with higher mortality in older patients who required admission to the hospital as emergencies. In addition, Seguchi et al.24 showed that activities of daily living before admission were associated with in-hospital death in very elderly patients with acute myocardial infarction. Furthermore, Kiani et al.25 reported that frailty including slow walking speed was associated with a composite event of all-cause death and re-hospitalization in patients undergoing transcatheter aortic valve replacement. In the present study, although we adjusted for pre-admission physical activities as the performance before admission, walking speed early in hospitalization may be associated with clinical outcomes because the patients had frailty and slow walking speed before admission.
Walking speed and rehabilitation in the early phase
Recently, the importance of early cardiac rehabilitation is increasing.11,26–28 Kaneko and Ueno29,30 reported that acute phase initiation of cardiac rehabilitation is associated with favourable clinical outcomes and improvement of activities of daily living in patients with acute HF from Japanese nationwide database. In addition, from the Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial, it is reported that tailored and progressive rehabilitation intervention improved short physical performance battery score consisted of walking speed, standing balance, and strength greater than usual care. Moreover, Tanaka et al.31 showed the association between short-term improvement of walking speed and favourable outcomes. In summarizing these reports, it may be affective for good clinical outcomes to provide rehabilitation programmes aimed at improving walking speed, especially in patients with slow walking speed at the initiation of walking.
Definition of early after admission
Several reports define early rehabilitation as the initiation within 2 or 3 days of hospital admission.29,30,32 In this study, the definition of early after admission was within 4 days of admission. In our results, the median initiation of ambulation was 2 days, which is almost consistent with previous studies. Also, there was no difference in the date of walking initiation between groups divided by tertile of walking speed. Therefore, we believe there is clinical value for measuring walking speed when first walking in the corridor (>10 m) after admission to the hospital. Furthermore, although the median length of hospital stay due to HF in Japan is reported to be 17 days,33 in countries with shorter hospital stays including the USA.34 walking speed at this phase may be considered as that at discharge. In fact, the median length of hospital stay in REHAB-HF trial is reported to be 4 days,27 suggesting that measuring walking speed at this time is useful for risk stratification.
Strength of the present research
The strength of this study is that it demonstrated that faster walking speeds in the early phase of admission are associated with favourable clinical outcomes, allowing risk stratification earlier after admission due to ADHF. The measurement of walking speed is simple and safe; in fact, we did not experience only a single adverse event by measuring walking speed. Therefore, we believe it is important to measure walking at the first corridor walk.
Limitations
The present study has several limitations in the present study. First, this was a relatively small retrospective study. In addition, because this was a single-centre study with a small staff, patients admitted on weekends may have delayed the start of rehabilitation, resulting in a delay in the measurement of walking speed. There were also many missing values, which could lead to a potentially high inclusion bias. Second, we had no data on the walking aids used when measuring walking speed. In addition, because of the early phase of admission, the patient may have received oxygen therapy, had a continuous intravenous infusion, and/or had a urinary catheter inserted, and these devices may have affected walking speed. Third, it is possible that the confounders used as adjustment variables are inadequate. In particular, only subjective activity information is available on physical function before admission is available. It is reported that physical dysfunction is associated with higher mortality in community-dwelling populations and that those with slower subjective walking speed are at a higher risk of incident heart failure, and our results may be influenced by these factors.35,36 Finally, this study was conducted in Japan only. Because of differences in insurance systems, the duration of hospitalization for HF treatment varies widely, and the timing of walking initiation may be different. It is necessary to investigate whether walking speed early after hospitalization is associated with outcomes in other countries as well.
Conclusions
A faster walking speed early after admission due to ADHF was found to be associated with favourable clinical outcomes. Our findings suggest that early measurement of walking speed may be useful for earlier risk stratification such as all-cause death and re-hospitalization due to HF.
Supplementary material
Supplementary material is available at European Journal of Cardiovascular Nursing online.
Author contributions
K.N.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, visualization, and writing—original draft. N.H.: conceptualization, data curation, formal analysis, investigation, methodology, validation, and writing—original draft. K.K.: conceptualization, methodology, resources, supervision, validation, and writing—review and editing. S.U.: conceptualization, formal analysis, methodology, resources, and writing—review and editing. T.N.: conceptualization, formal analysis, methodology, resources, and writing—review and editing. K.U.: conceptualization, formal analysis, methodology, resources, and writing—review and editing. K.H.: conceptualization, methodology, methodology, resources, supervision, and writing—review and editing. E.M.: conceptualization, methodology, resources, supervision, and writing—review and editing. A.M.: conceptualization, methodology, project administration, supervision, and writing—review and editing. M.Y.-T.: conceptualization, methodology, project administration, supervision, and writing—review and editing. J.A.: conceptualization, methodology, project administration, supervision, and writing—review and editing.
Funding
This study was supported by a Grant-in-Aid (JSPS KAKENHI Grant Number: JP 19K19884) from the Japan Society for the Promotion of Science.
Data availability
The data used in this article cannot be shared publicly in view of the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
References
Author notes
Conflict of interest: None declared in all authors.




Comments