-
PDF
- Split View
-
Views
-
Cite
Cite
Sandra B Lauck, Maggie Yu, Carrie Bancroft, Britt Borregaard, Jopie Polderman, Anna L Stephenson, Eric Durand, Mariama Akodad, David Meier, Holly Andrews, Leslie Achtem, Erin Tang, David A Wood, Janarthanan Sathananthan, John G Webb, Early mobilization after transcatheter aortic valve implantation: observational cohort study, European Journal of Cardiovascular Nursing, Volume 23, Issue 3, April 2024, Pages 296–304, https://doi.org/10.1093/eurjcn/zvad081
Close - Share Icon Share
Abstract
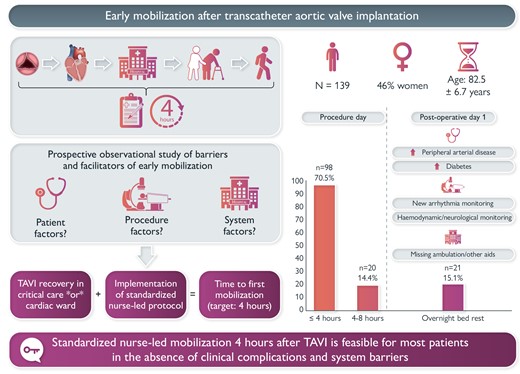
Early mobilization is associated with improved outcomes in hospitalized older patients. We sought to determine the effect of a nurse-led protocol on mobilization 4 h after transfemoral transcatheter aortic valve implantation (TAVI) across different units of care.
We conducted a prospective observational cohort single-centre study of consecutive patients. We implemented a standardized protocol for safe early recovery and progressive mobilization in the critical care and cardiac telemetry units. We measured the time to first mobilization and conducted descriptive statistics to identify patient and system barriers to timely ambulation. We recruited 139 patients (82.5 years, SD = 6.7; 46% women). At baseline, patients who were mobilized early (≤4 h) and late (>4 h) did not differ, except for higher rates of diabetes (25.5% vs. 43.9%, P = 0.032) and peripheral arterial disease (8.2% vs. 26.8%, P = 0.003) in the late mobilization group. The median time to mobilization was 4 h [inter-quartile range (IQR) 3.25, 4]; 98 patients (70.5%) were mobilized successfully after 4 h of bedrest; 118 (84.9%) were walking by the evening of the procedure (<8 h bedrest); and 21 (15.1%) were on bedrest overnight and mobilized the following day. Primary reasons for overnight bedrest were arrhythmia monitoring (n = 10, 7.2%) and haemodynamic and/or neurological instability (n = 6, 4.3%); six patients (4.3%) experienced delayed ambulation due to system issues. Procedure location in the hybrid operating room and transfer to critical care were associated with longer bedrest times.
Standardized nurse-led mobilization 4 h after TF TAVI is feasible in the absence of clinical complications and system barriers.
There is wide variation of bedrest time after transcatheter aortic valve implantation (TAVI) and limited evidence to support cardiovascular nursing practice.
The study provides novel evidence cardiac telemetry and critical care nurses can safely mobilise most patients 4 h after TAVI in the absence of complications.
Consistent early mobilisation may contribute to the avoidance of in-hospital complications and improved outcomes of older patients with valvular heart disease.
Introduction
Transcatheter aortic valve implantation (TAVI) is an established treatment for severe symptomatic aortic stenosis across surgical risk profiles. Increasingly, TAVI has surpassed surgical aortic valve replacement (SAVR) as the dominant treatment option across international regions.1,2 Clinical outcomes continue to improve, resulting in growing recommendations in international guidelines.3,4 There is increasing evidence that TAVI is associated with accelerated improvement in patient-reported outcomes5 and cost savings.6 The implementation of a minimalist procedure and streamlined clinical pathway has facilitated rapid recovery, mitigation of in-hospital complications, and safe next-day discharge home after transfemoral (TF) TAVI.7,8
In spite of the decreasing incidence of vascular complications, the availability of lower profile delivery systems, and improved procedural approaches, TF TAVI patients require a period of bedrest to achieve femoral artery access haemostasis. In this primarily older valvular heart disease patient population, extended bedrest can have a detrimental impact on functional status and cause hospital-associated disability (HAD).9 Immobility-induced HAD can be an unintended consequence of admission. Healthy and well-nourished individuals who are immobilized exhibit signs of skeletal muscle atrophy within 72 h, while 50% of disability among older adults occurs during hospitalization.10–12 The ensuing loss of muscle mass and increased risk of falls, delirium, and pneumonia are associated with a longer length of stay, higher costs, and an increased requirement for institutionalization after discharge.9,13,14 Across regions, healthcare organizations have adopted practices and cultures of care structured to promote immobility, the use of tethering devices (intravenous or urinary catheters, cardiac monitoring cables), and patients’ reliance on others for basic activities of daily living (toileting, hygiene, and feeding).15 Unnecessarily extended bedrest and the cascade of immobility-associated issues (e.g. pain, sleep disturbance, delirium, urinary retention/incontinence, and falls) can rapidly impact older cardiac patients’ functional capacity and jeopardize procedural success.16,17
The duration of bedrest after TF TAVI varies across programmes; this may be associated with protocols developed in earlier eras of larger delivery devices or due to the limited evidence about the optimal time to ambulation to guide nursing practice. In this context, we sought to determine the effect of a nurse-led protocol on mobilization 4 h after TF TAVI across different units of care and to identify patient- and system-level barriers to improving standardization of care.
Methods
Study design
We conducted a prospective observational cohort study of consecutive patients who had successful TF TAVI in a single centre in 2020. We included elective out-patients and in-patients who were independent with mobilization or required a walking aid (cane and walker); we excluded patients who ambulated with a wheelchair or presented emergently with severe haemodynamic instability. We followed the STROBE guidelines for reporting observational cohort studies (https://www.equator-network.org/reporting-guidelines/strobe/).
The procedure location was a cardiac catheterization laboratory (CCL) or a hybrid operating room (HOR) based on availability or procedural risk. Standardized peri-procedure practices pertinent to mobilization included the following: ultrasound-guided vascular access and percutaneous suture-based closure (with confirmation of access closure by angiography in selective cases when appropriate), default strategy of local anaesthesia and mild sedation, minimal use of invasive lines, removal of a temporary pacemaker at the end of the procedure, peri-procedure monitoring of activated clotting time, and use of non-compressive dressings on the access sites.18 The recovery pathways included either 2 h admission to the cardiac short-stay unit adjacent to CCL and transfer to cardiac telemetry or admission to the cardiac intensive care unit (CICU), with or without transfer to cardiac telemetry prior to discharge.19 The selection of the recovery location was driven by bed availability and patient acuity. Patients who required on-going monitoring of cardiac rhythm, haemodynamics, and/or neurological status were admitted or transferred to the CICU for close and constant monitoring (Figure 1). In most cases, nursing assignments were one registered nurse (RN) for four to five patients in cardiac telemetry, and one RN for one to three patients in cardiac short-stay and CICUs. Discharge target was the morning after the procedure.
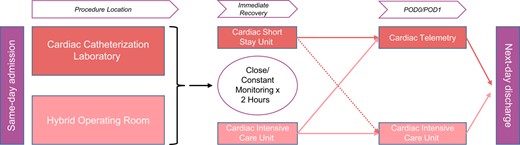
Location of post-procedure care after TF TAVR in cardiac catheterization laboratory or hybrid operating room.
Mobilization protocol
In collaboration with the CHU Rouen (France) TAVI programme, we co-developed a multidisciplinary protocol to standardize practice in all clinical areas and to integrate early mobilization as a key intervention in the overall TF TAVI clinical pathway. The nursing care standard outlined essential components of communication at the time of admission between the implanting and post-procedure teams to report on vascular access and closure and other pertinent clinical information to promote continuity of care at the onset of recovery. Close monitoring of the vascular access site and peripheral circulation matched the timing of on-going haemodynamic, cardiac rhythm, and neurological assessments to ensure patient safety. Following an initial 2 h period of supine positioning, the head of the bed was raised to 30° to promote patient comfort. In the absence of complications and based on nurses’ assessments of haemostasis and clinical status, patients were assisted to stand and walk 4 h after the end of the procedure, with the goal of ambulating to the toilet during the first mobilization episode. Standard prescribers’ orders indicated directives to sit in a chair for supper and mobilize twice on the day of the procedure. Nurses assessed patients’ return to baseline ambulation in the morning following the procedure to confirm eligibility for next-day discharge. The implanting physician team was readily available to address nurses’ concerns about vascular access site complications and other issues to achieve the mobilization targets and avoid variances from the clinical pathway. We outline the details of the Vancouver–Rouen early mobilization protocol in Table 1.
Vancouver–Rouen early mobilization protocol to achieve 4 h ambulation after TF TAVI
| Peri-procedure: medical techniques to facilitate early mobilization |
|
| Communication: report from peri-procedure to post-procedure team |
|
| Monitoring and education: post-procedure hours 0–4 |
|
| First mobilization after 4 h bedrest |
|
| Procedure day targets |
|
| Post-procedure day 1 targets |
|
| Peri-procedure: medical techniques to facilitate early mobilization |
|
| Communication: report from peri-procedure to post-procedure team |
|
| Monitoring and education: post-procedure hours 0–4 |
|
| First mobilization after 4 h bedrest |
|
| Procedure day targets |
|
| Post-procedure day 1 targets |
|
Vancouver–Rouen early mobilization protocol to achieve 4 h ambulation after TF TAVI
| Peri-procedure: medical techniques to facilitate early mobilization |
|
| Communication: report from peri-procedure to post-procedure team |
|
| Monitoring and education: post-procedure hours 0–4 |
|
| First mobilization after 4 h bedrest |
|
| Procedure day targets |
|
| Post-procedure day 1 targets |
|
| Peri-procedure: medical techniques to facilitate early mobilization |
|
| Communication: report from peri-procedure to post-procedure team |
|
| Monitoring and education: post-procedure hours 0–4 |
|
| First mobilization after 4 h bedrest |
|
| Procedure day targets |
|
| Post-procedure day 1 targets |
|
Endpoints and data sources
The primary outcome of the study was time to mobilization; we defined early mobilization from the end of the procedure (successful vascular closure) to 4 h. We sought to describe the primary reasons for prolonged bedrest and explore differences in characteristics between patients who achieved or did not achieve the early mobilization target. The research question was raised by the nurses at point-of-care in the Providence Health Care Practice-Based Research Challenge, an annual competition that equips nurses with additional research training and mentorship to address a pressing clinical issue.20 Supported by an embedded cardiovascular nurse clinician scientist, nurses formed a core group of co-investigators who led data collection. Nurses prospectively collected data to capture the details of patients’ clinical journeys with a focus on their mobilization; data were further augmented with a review of medical records. The University of British Columbia’s Research Ethics Board granted study approval with an exemption to obtain individual consent (certificate H19–03049).
Statistical methods
We conducted a descriptive analysis of the findings. Results are presented as the mean (standard deviation) or median [inter-quartile range (IQR)] for continuous variables and as a number (percentage) for categorical data. Student’s t-test was used to compare continuous variables, and χ2 and Fischer exact tests were used to compare categorical variables. All analyses were performed in SAS version 9.4 (SAS institute).
Results
The study cohort included 139 patients, mean age 82.5 years (SD = 6.7) and 46% women. No patients were excluded. The mean Society of Thoracic Surgeon (STS) predicted mortality score was 3.2 (SD = 1.9); patients presented with high comorbidity burden, including chronic lung disease (28.1%), atrial fibrillation (41.7%), prior stroke (13.7%), and impaired renal function (eGFR < 30 mL/min: 45.3%); 51.8% presented with NYHA functional class III/IV. At baseline, there were no significant differences in characteristics between patients who were mobilized early (≤4 h) and late (>4 h), except for a higher incidence of diabetes [25.5% vs. 43.9%, 95% CI of absolute difference (1%, 36%), P = 0.032] and peripheral arterial disease [8.2% vs. 26.8%, 95% CI of absolute difference (4%, 33%), P = 0.003] in the late mobilization group (Table 2).
Patient characteristics and procedural details by time to first mobilization
| . | All (n = 139) . | Mobilization ≤ 4 h (n = 98) . | Mobilization > 4 h (n = 41) . | P-value . |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (mean ± SD) | 82.5 ± 6.7 | 82.2 ± 7.2 | 83.3 ± 5.4 | 0.376 |
| Male | 75 (54.0%) | 49 (50.0%) | 26 (63.4%) | 0.148 |
| STS score (mean ± SD) | 3.2 ± 1.9 | 3.2 ± 2 | 3.1 ± 1.8 | 0.705 |
| Body mass index (mean ± SD) | 27.4 ± 5.4 | 27.3 ± 4.9 | 27.6 ± 6.5 | 0.730 |
| Hypertension | 126 (90.7%) | 89 (90.8%) | 37 (90.2%) | 0.916 |
| NYHA III/IV | 72 (51.8%) | 53 (54.1%) | 19 (46.3%) | 0.405 |
| LVEF (%, mean ± SD) | 57.1 ± 11.7 | 56.6 ± 11.8 | 58.3 ± 11.3 | 0.441 |
| LVEF < 35% | 9 (6.5%) | 7 (7.1%) | 2 (4.9%) | 0.621 |
| Peripheral arterial disease | 19 (13.7%) | 8 (8.2%) | 11 (26.8%) | 0.003 |
| Atrial fibrillation | 58 (41.7%) | 38 (38.8%) | 20 (48.8%) | 0.275 |
| Stroke | 19 (13.7%) | 11 (11.2%) | 8 (19.5%) | 0.195 |
| Current smoker | 8 (5.8%) | 7 (7.1%) | 1 (2.4%) | 0.278 |
| Dyslipidaemia | 85 (63.0%) | 60 (62.5%) | 25 (64.1%) | 0.861 |
| Chronic lung disease | 39 (28.1%) | 27 (27.6%) | 12 (29.3%) | 0.837 |
| Diabetes | 43 (30.9%) | 25 (25.5%) | 18 (43.9%) | 0.032 |
| Dialysis | 2 (1.4%) | 2 (2.0%) | 0 (0.0%) | 0.357 |
| eGFR (mL/min, mean ± SD) | 61.5 ± 19.9 | 61.5 ± 19.9 | 62 ± 20.4 | 0.333 |
| eGFR < 30 mL/min | 63 (45.3%) | 8 (8.2%) | 2 (4.9%) | 0.494 |
| Pacemaker | 18 (13.0%) | 16 (16.3%) | 3 (7.3%) | 0.159 |
| Percutaneous coronary intervention | 23 (16.6%) | 18 (18.4%) | 5 (12.2%) | 0.372 |
| Coronary artery bypass graft | 13 (9.4%) | 8 (8.2%) | 5 (12.2%) | 0.457 |
| Surgical aortic valve replacement | 11 (7.9%) | 10 (10.2%) | 1 (2.4%) | 0.122 |
| Urgent in-patient | 15 (10.8%) | 11 (11.2%) | 4 (9.8%) | 0.799 |
| Procedural details | ||||
| Procedure location | <0.001 | |||
| Cardiac cath lab | 111 (79.9%) | 86 (87.8%) | 25 (61.0%) | |
| Hybrid operating room | 28 (20.1%) | 12 (12.2%) | 16 (39.0%) | |
| Procedure times (min; mean ± SD) | ||||
| Entry to exit | 69.7 ± 28.3 | 65.7 ± 19.1 | 79.2 ± 41.6 | 0.010 |
| Incision to closure | 52.6 ± 25.5 | 49.7 ± 16.9 | 59.4 ± 38.5 | 0.040 |
| Anaesthesia strategy | ||||
| Local anaesthesia only | 29 (20.9%) | 20 (20.4%) | 9 (22.0%) | 0.838 |
| Local anaesthesia + sedation | 106 (76.3%) | 75 (76.5%) | 31 (75.6%) | 0.907 |
| General anaesthesia | 4 (2.9%) | 3 (3.1%) | 1 (2.4%) | 0.841 |
| TAVR device | 0.635 | |||
| Balloon expandable | 134 (96.4%) | 94 (95.9%) | 40 (97.6%) | |
| Other | 5 (3.6%) | 4 (4.1%) | 1 (2.4%) | |
| . | All (n = 139) . | Mobilization ≤ 4 h (n = 98) . | Mobilization > 4 h (n = 41) . | P-value . |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (mean ± SD) | 82.5 ± 6.7 | 82.2 ± 7.2 | 83.3 ± 5.4 | 0.376 |
| Male | 75 (54.0%) | 49 (50.0%) | 26 (63.4%) | 0.148 |
| STS score (mean ± SD) | 3.2 ± 1.9 | 3.2 ± 2 | 3.1 ± 1.8 | 0.705 |
| Body mass index (mean ± SD) | 27.4 ± 5.4 | 27.3 ± 4.9 | 27.6 ± 6.5 | 0.730 |
| Hypertension | 126 (90.7%) | 89 (90.8%) | 37 (90.2%) | 0.916 |
| NYHA III/IV | 72 (51.8%) | 53 (54.1%) | 19 (46.3%) | 0.405 |
| LVEF (%, mean ± SD) | 57.1 ± 11.7 | 56.6 ± 11.8 | 58.3 ± 11.3 | 0.441 |
| LVEF < 35% | 9 (6.5%) | 7 (7.1%) | 2 (4.9%) | 0.621 |
| Peripheral arterial disease | 19 (13.7%) | 8 (8.2%) | 11 (26.8%) | 0.003 |
| Atrial fibrillation | 58 (41.7%) | 38 (38.8%) | 20 (48.8%) | 0.275 |
| Stroke | 19 (13.7%) | 11 (11.2%) | 8 (19.5%) | 0.195 |
| Current smoker | 8 (5.8%) | 7 (7.1%) | 1 (2.4%) | 0.278 |
| Dyslipidaemia | 85 (63.0%) | 60 (62.5%) | 25 (64.1%) | 0.861 |
| Chronic lung disease | 39 (28.1%) | 27 (27.6%) | 12 (29.3%) | 0.837 |
| Diabetes | 43 (30.9%) | 25 (25.5%) | 18 (43.9%) | 0.032 |
| Dialysis | 2 (1.4%) | 2 (2.0%) | 0 (0.0%) | 0.357 |
| eGFR (mL/min, mean ± SD) | 61.5 ± 19.9 | 61.5 ± 19.9 | 62 ± 20.4 | 0.333 |
| eGFR < 30 mL/min | 63 (45.3%) | 8 (8.2%) | 2 (4.9%) | 0.494 |
| Pacemaker | 18 (13.0%) | 16 (16.3%) | 3 (7.3%) | 0.159 |
| Percutaneous coronary intervention | 23 (16.6%) | 18 (18.4%) | 5 (12.2%) | 0.372 |
| Coronary artery bypass graft | 13 (9.4%) | 8 (8.2%) | 5 (12.2%) | 0.457 |
| Surgical aortic valve replacement | 11 (7.9%) | 10 (10.2%) | 1 (2.4%) | 0.122 |
| Urgent in-patient | 15 (10.8%) | 11 (11.2%) | 4 (9.8%) | 0.799 |
| Procedural details | ||||
| Procedure location | <0.001 | |||
| Cardiac cath lab | 111 (79.9%) | 86 (87.8%) | 25 (61.0%) | |
| Hybrid operating room | 28 (20.1%) | 12 (12.2%) | 16 (39.0%) | |
| Procedure times (min; mean ± SD) | ||||
| Entry to exit | 69.7 ± 28.3 | 65.7 ± 19.1 | 79.2 ± 41.6 | 0.010 |
| Incision to closure | 52.6 ± 25.5 | 49.7 ± 16.9 | 59.4 ± 38.5 | 0.040 |
| Anaesthesia strategy | ||||
| Local anaesthesia only | 29 (20.9%) | 20 (20.4%) | 9 (22.0%) | 0.838 |
| Local anaesthesia + sedation | 106 (76.3%) | 75 (76.5%) | 31 (75.6%) | 0.907 |
| General anaesthesia | 4 (2.9%) | 3 (3.1%) | 1 (2.4%) | 0.841 |
| TAVR device | 0.635 | |||
| Balloon expandable | 134 (96.4%) | 94 (95.9%) | 40 (97.6%) | |
| Other | 5 (3.6%) | 4 (4.1%) | 1 (2.4%) | |
eGFR, estimated glomerular filtration; LVEF, left ventricular ejection fraction; STS, Society of Thoracic Surgeon predicted risk of mortality score.
Significance of bold values represents P < .05.
Patient characteristics and procedural details by time to first mobilization
| . | All (n = 139) . | Mobilization ≤ 4 h (n = 98) . | Mobilization > 4 h (n = 41) . | P-value . |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (mean ± SD) | 82.5 ± 6.7 | 82.2 ± 7.2 | 83.3 ± 5.4 | 0.376 |
| Male | 75 (54.0%) | 49 (50.0%) | 26 (63.4%) | 0.148 |
| STS score (mean ± SD) | 3.2 ± 1.9 | 3.2 ± 2 | 3.1 ± 1.8 | 0.705 |
| Body mass index (mean ± SD) | 27.4 ± 5.4 | 27.3 ± 4.9 | 27.6 ± 6.5 | 0.730 |
| Hypertension | 126 (90.7%) | 89 (90.8%) | 37 (90.2%) | 0.916 |
| NYHA III/IV | 72 (51.8%) | 53 (54.1%) | 19 (46.3%) | 0.405 |
| LVEF (%, mean ± SD) | 57.1 ± 11.7 | 56.6 ± 11.8 | 58.3 ± 11.3 | 0.441 |
| LVEF < 35% | 9 (6.5%) | 7 (7.1%) | 2 (4.9%) | 0.621 |
| Peripheral arterial disease | 19 (13.7%) | 8 (8.2%) | 11 (26.8%) | 0.003 |
| Atrial fibrillation | 58 (41.7%) | 38 (38.8%) | 20 (48.8%) | 0.275 |
| Stroke | 19 (13.7%) | 11 (11.2%) | 8 (19.5%) | 0.195 |
| Current smoker | 8 (5.8%) | 7 (7.1%) | 1 (2.4%) | 0.278 |
| Dyslipidaemia | 85 (63.0%) | 60 (62.5%) | 25 (64.1%) | 0.861 |
| Chronic lung disease | 39 (28.1%) | 27 (27.6%) | 12 (29.3%) | 0.837 |
| Diabetes | 43 (30.9%) | 25 (25.5%) | 18 (43.9%) | 0.032 |
| Dialysis | 2 (1.4%) | 2 (2.0%) | 0 (0.0%) | 0.357 |
| eGFR (mL/min, mean ± SD) | 61.5 ± 19.9 | 61.5 ± 19.9 | 62 ± 20.4 | 0.333 |
| eGFR < 30 mL/min | 63 (45.3%) | 8 (8.2%) | 2 (4.9%) | 0.494 |
| Pacemaker | 18 (13.0%) | 16 (16.3%) | 3 (7.3%) | 0.159 |
| Percutaneous coronary intervention | 23 (16.6%) | 18 (18.4%) | 5 (12.2%) | 0.372 |
| Coronary artery bypass graft | 13 (9.4%) | 8 (8.2%) | 5 (12.2%) | 0.457 |
| Surgical aortic valve replacement | 11 (7.9%) | 10 (10.2%) | 1 (2.4%) | 0.122 |
| Urgent in-patient | 15 (10.8%) | 11 (11.2%) | 4 (9.8%) | 0.799 |
| Procedural details | ||||
| Procedure location | <0.001 | |||
| Cardiac cath lab | 111 (79.9%) | 86 (87.8%) | 25 (61.0%) | |
| Hybrid operating room | 28 (20.1%) | 12 (12.2%) | 16 (39.0%) | |
| Procedure times (min; mean ± SD) | ||||
| Entry to exit | 69.7 ± 28.3 | 65.7 ± 19.1 | 79.2 ± 41.6 | 0.010 |
| Incision to closure | 52.6 ± 25.5 | 49.7 ± 16.9 | 59.4 ± 38.5 | 0.040 |
| Anaesthesia strategy | ||||
| Local anaesthesia only | 29 (20.9%) | 20 (20.4%) | 9 (22.0%) | 0.838 |
| Local anaesthesia + sedation | 106 (76.3%) | 75 (76.5%) | 31 (75.6%) | 0.907 |
| General anaesthesia | 4 (2.9%) | 3 (3.1%) | 1 (2.4%) | 0.841 |
| TAVR device | 0.635 | |||
| Balloon expandable | 134 (96.4%) | 94 (95.9%) | 40 (97.6%) | |
| Other | 5 (3.6%) | 4 (4.1%) | 1 (2.4%) | |
| . | All (n = 139) . | Mobilization ≤ 4 h (n = 98) . | Mobilization > 4 h (n = 41) . | P-value . |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (mean ± SD) | 82.5 ± 6.7 | 82.2 ± 7.2 | 83.3 ± 5.4 | 0.376 |
| Male | 75 (54.0%) | 49 (50.0%) | 26 (63.4%) | 0.148 |
| STS score (mean ± SD) | 3.2 ± 1.9 | 3.2 ± 2 | 3.1 ± 1.8 | 0.705 |
| Body mass index (mean ± SD) | 27.4 ± 5.4 | 27.3 ± 4.9 | 27.6 ± 6.5 | 0.730 |
| Hypertension | 126 (90.7%) | 89 (90.8%) | 37 (90.2%) | 0.916 |
| NYHA III/IV | 72 (51.8%) | 53 (54.1%) | 19 (46.3%) | 0.405 |
| LVEF (%, mean ± SD) | 57.1 ± 11.7 | 56.6 ± 11.8 | 58.3 ± 11.3 | 0.441 |
| LVEF < 35% | 9 (6.5%) | 7 (7.1%) | 2 (4.9%) | 0.621 |
| Peripheral arterial disease | 19 (13.7%) | 8 (8.2%) | 11 (26.8%) | 0.003 |
| Atrial fibrillation | 58 (41.7%) | 38 (38.8%) | 20 (48.8%) | 0.275 |
| Stroke | 19 (13.7%) | 11 (11.2%) | 8 (19.5%) | 0.195 |
| Current smoker | 8 (5.8%) | 7 (7.1%) | 1 (2.4%) | 0.278 |
| Dyslipidaemia | 85 (63.0%) | 60 (62.5%) | 25 (64.1%) | 0.861 |
| Chronic lung disease | 39 (28.1%) | 27 (27.6%) | 12 (29.3%) | 0.837 |
| Diabetes | 43 (30.9%) | 25 (25.5%) | 18 (43.9%) | 0.032 |
| Dialysis | 2 (1.4%) | 2 (2.0%) | 0 (0.0%) | 0.357 |
| eGFR (mL/min, mean ± SD) | 61.5 ± 19.9 | 61.5 ± 19.9 | 62 ± 20.4 | 0.333 |
| eGFR < 30 mL/min | 63 (45.3%) | 8 (8.2%) | 2 (4.9%) | 0.494 |
| Pacemaker | 18 (13.0%) | 16 (16.3%) | 3 (7.3%) | 0.159 |
| Percutaneous coronary intervention | 23 (16.6%) | 18 (18.4%) | 5 (12.2%) | 0.372 |
| Coronary artery bypass graft | 13 (9.4%) | 8 (8.2%) | 5 (12.2%) | 0.457 |
| Surgical aortic valve replacement | 11 (7.9%) | 10 (10.2%) | 1 (2.4%) | 0.122 |
| Urgent in-patient | 15 (10.8%) | 11 (11.2%) | 4 (9.8%) | 0.799 |
| Procedural details | ||||
| Procedure location | <0.001 | |||
| Cardiac cath lab | 111 (79.9%) | 86 (87.8%) | 25 (61.0%) | |
| Hybrid operating room | 28 (20.1%) | 12 (12.2%) | 16 (39.0%) | |
| Procedure times (min; mean ± SD) | ||||
| Entry to exit | 69.7 ± 28.3 | 65.7 ± 19.1 | 79.2 ± 41.6 | 0.010 |
| Incision to closure | 52.6 ± 25.5 | 49.7 ± 16.9 | 59.4 ± 38.5 | 0.040 |
| Anaesthesia strategy | ||||
| Local anaesthesia only | 29 (20.9%) | 20 (20.4%) | 9 (22.0%) | 0.838 |
| Local anaesthesia + sedation | 106 (76.3%) | 75 (76.5%) | 31 (75.6%) | 0.907 |
| General anaesthesia | 4 (2.9%) | 3 (3.1%) | 1 (2.4%) | 0.841 |
| TAVR device | 0.635 | |||
| Balloon expandable | 134 (96.4%) | 94 (95.9%) | 40 (97.6%) | |
| Other | 5 (3.6%) | 4 (4.1%) | 1 (2.4%) | |
eGFR, estimated glomerular filtration; LVEF, left ventricular ejection fraction; STS, Society of Thoracic Surgeon predicted risk of mortality score.
Significance of bold values represents P < .05.
Procedural details
Patients were treated using local anaesthesia only (n = 29, 20.9%) or with minimal/conscious sedation (n = 106, 76.3%). TAVI was primarily performed with a balloon-expandable device (n = 134, 96.4%) in a CCL [n = 111, 79.9% with 95% CI (73%, 87%)] or HOR [n = 28, 20.1% with 95% CI (13%, 27%)]; the use of the hybrid room was associated with delayed mobilization [absolute difference of 27%, 95% CI (11%, 43%), P < 0.001]. All patients had successful percutaneous vascular closure of the device delivery and the contralateral arterial access sites. At the end of the procedure, all patients had a radial artery monitoring line in place that was removed within the first hour of recovery and 8 (5.9%) had a femoral transvenous pacing line left in situ. Central venous line or urinary catheters were not used. Mean procedure times from entry to exit and from skin incision to closure were 69.7 (SD = 28.3) and 52.6 (SD = 25.5) min, respectively. Anaesthesia strategy was not associated with time to mobilization (Table 2). At the end of the procedure, 101 patients (87.1%) were transferred to the cardiac short-stay unit and 38 (27.3%) were admitted to the CICU.
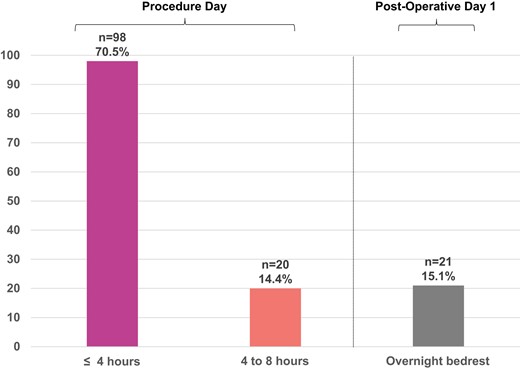
Time to mobilization
The median time to mobilization in the study cohort was 4 h (IQR 3.25, 4); 98 patients (70.5%) were mobilized successfully after 4 h of bedrest on post-operative day 0 (POD0); 118 (84.9%) were walking by the evening of the procedure (<8 h bedrest); and 21 (15.1%) were on bedrest overnight and mobilized on POD1 (Figure 2). Early mobilization was achieved in 87.1% (n = 88) of patients admitted to cardiac telemetry and 65.8% (n = 38) of patients admitted to the CICU (P < 0.001). The highest level of mobilization achieved in the same-day mobilization group was walking outside of the room in the hallway of the unit (n = 63, 53.3%) and ambulation to the toilet (n = 48, 40.6%), while seven patients sat at the edge of the bed (n = 4, 3.4%) or transferred to a chair (n = 3, 2.5%) (Figure 3).
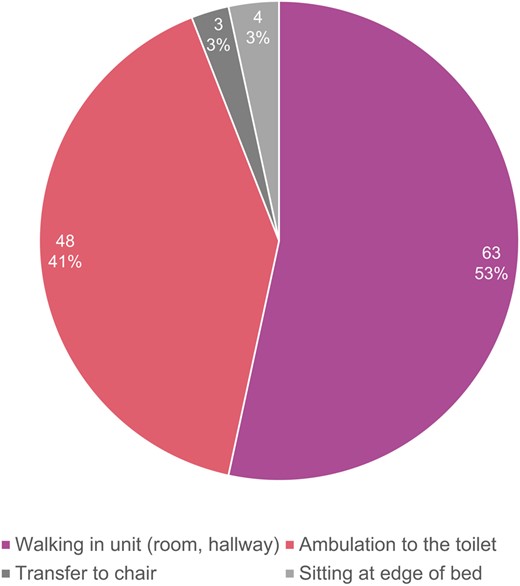
Highest mobilization activity achieved on post-operative day 0 (n = 118).
Barriers to early mobilization
The primary indication for the 41 (29.5%) patients who experienced delayed mobilization (>4 h) on the day of their procedure was attributed to monitoring of vascular access (n = 14, 10.1%); of these, 12 (8.7%) patients achieved successful haemostasis and mobilization with 4 to 8 h of the procedure, while two (1.4%) patients required overnight bedrest. Among the 21 (15.1%) patients who required overnight bedrest, 10 (7.2%) were closely monitored for heart rhythm changes related to a new conduction delay; among these patients, three (2.6%) required a new permanent pacemaker. In addition, six (4.3%) patients had haemodynamic and/or neurological issues requiring overnight bedrest and monitoring. Systems issues on the procedure day were associated with delayed ambulation in seven (5.0%) patients, including nursing workload (n = 2, 1.4%), physician order for longer mobilization not explained by patient status (n = 2, 1.4%), and unavailable mobility or other aid (n = 3, 2.6%) (Table 3).
| . | Delayed mobilization on POD0 . | Overnight bedrest . |
|---|---|---|
| (n = 41, 29.5%) . | (n = 21, 15.1%) . | |
| Unstable patient status | ||
| Monitoring of vascular access issues | 14 | 2 |
| Monitoring of heart rhythm for assessment of new conduction delay | 13 | 10 |
| Monitoring of haemodynamic and/or neurological status | 6 | 6 |
| Systems issues | ||
| Nursing workload | 2 | 0 |
| MD order not explained by patient status | 2 | 0 |
| Missing mobility or other aids | 3 | 3 |
| . | Delayed mobilization on POD0 . | Overnight bedrest . |
|---|---|---|
| (n = 41, 29.5%) . | (n = 21, 15.1%) . | |
| Unstable patient status | ||
| Monitoring of vascular access issues | 14 | 2 |
| Monitoring of heart rhythm for assessment of new conduction delay | 13 | 10 |
| Monitoring of haemodynamic and/or neurological status | 6 | 6 |
| Systems issues | ||
| Nursing workload | 2 | 0 |
| MD order not explained by patient status | 2 | 0 |
| Missing mobility or other aids | 3 | 3 |
| . | Delayed mobilization on POD0 . | Overnight bedrest . |
|---|---|---|
| (n = 41, 29.5%) . | (n = 21, 15.1%) . | |
| Unstable patient status | ||
| Monitoring of vascular access issues | 14 | 2 |
| Monitoring of heart rhythm for assessment of new conduction delay | 13 | 10 |
| Monitoring of haemodynamic and/or neurological status | 6 | 6 |
| Systems issues | ||
| Nursing workload | 2 | 0 |
| MD order not explained by patient status | 2 | 0 |
| Missing mobility or other aids | 3 | 3 |
| . | Delayed mobilization on POD0 . | Overnight bedrest . |
|---|---|---|
| (n = 41, 29.5%) . | (n = 21, 15.1%) . | |
| Unstable patient status | ||
| Monitoring of vascular access issues | 14 | 2 |
| Monitoring of heart rhythm for assessment of new conduction delay | 13 | 10 |
| Monitoring of haemodynamic and/or neurological status | 6 | 6 |
| Systems issues | ||
| Nursing workload | 2 | 0 |
| MD order not explained by patient status | 2 | 0 |
| Missing mobility or other aids | 3 | 3 |
Discharge and disposition
In the early mobilization group, 81 patients (82.7%) were discharged on POD1 and all returned home. In the late mobilization group, 20 patients (14.4%) were eligible for next-day discharge; overall, 101 (72.7%) patients were discharged in POD1.
Discussion
In this prospective study of time to mobilization after TF TAVI, we found that a standardized nurse-led protocol aiming at ambulating patients after 4 h of bedrest was successfully implemented. The need for monitoring of vascular access issues, cardiac rhythm, and/or haemodynamic or neurological status was associated with longer bedrest time; procedure and recovery location pathways were associated with time to mobilization. In addition, health system issues related to staffing, communication, and timely availability of mobilization equipment caused delays in target mobilization. In the absence of these factors, a standardized protocol of 4 h mobilization after TF TAVI was successfully implemented in critical care and cardiac telemetry units. The study offers important new evidence to establish best practices, promote the standardization of post-procedure TAVI nursing protocols, and promote the adoption of a streamlined clinical pathway to optimize outcomes and help mitigate iatrogenic risks during patients’ short admissions.
Bedrest after cardiac procedures
To our knowledge, the only previous study of early mobilization (4–6 h) after TAVI excluded 55% of patients due to various reasons, including vascular access challenges (39%) and arrhythmias (7%).21 In contrast, our study included all consecutive elective out-patients and in-patients who ambulated without a wheelchair and were haemodynamically stable at baseline. Findings can be further examined in the context of the large body of research that has previously examined time to mobilization following other minimally invasive cardiac diagnostic and interventional procedures. In a recent meta-analysis of bedrest duration and complications in over 9000 patients recovering from TF cardiac catheterization, short bedrest (2–2.9 h) was not associated with complications (haematoma or bleeding) and patients immobilized for longer duration (>12 h) were likely to experience significantly more pain.22 In randomized comparisons of 2 vs. 4 h23 and immediate mobilization vs. 2 h bedrest after percutaneous coronary intervention,24 patients who were mobilized earlier did not sustain more complications than patients who remained on bedrest longer. In contrast, patients recovering from cardiac electronic device implantation placed on post-operative bedrest for 24 h experienced high rates of severe pain, sleep disturbance, delirium, and urinary retention that were associated with significantly longer lengths of stay. Further research is needed to guide the management of the larger profile TAVI delivery devices to further tailor time to mobilization and stratify patients’ risks for these adverse outcomes.
Interventions aimed at addressing challenges to mobilization reported by patients—including their health status (weakness, pain, and fatigue), having an intravenous line or other invasive device, fear of falling, and a lack of staff to help with ambulation and activities—result in shorter length of stay, increased rates of discharge home, and lower costs.13,25,26 Hospital cultures that shift the responsibility for mobilizing patients to physiotherapy alone fail to leverage the expertise of nursing, the collective impact of the whole team, and patients’ families towards the imperative of rapid reconditioning. The onus placed on the availability of the physiotherapist to initiate safe mobilization after TAVI can also have the unintended consequence of patients and their families adopting the sick role—the detrimental disempowerment to attend to activities of daily living, expectations of a complex recovery trajectory, and the gradual loss of function.27
Strengthening the TAVI clinical pathway
In the first decade of TAVI innovation, clinicians and researchers focused their attention on the development of improved devices, multimodality assessment, case selection, and procedural approaches.28,29 These collective efforts resulted in TAVI rapidly becoming established as a safe and effective treatment option for people with symptomatic severe aortic stenosis with surgical profiles ranging from prohibitive to low. Today, TAVI has surpassed SAVR as the preferred treatment for AS in multiple international jurisdictions.30,31 There is now growing evidence supporting the use of a streamlined clinical pathway for TAVI patients to optimize clinical and patient-reported outcomes, health service utilization, safe early discharge home, and costs.8,32,33 Our study adds to this evidence and strengthens the current focus on the scrutiny of the inter-related components of pre-, peri-, and post-procedure best practices guiding TAVI care.
Successful early mobilization is the end result of multiple interconnected variables. Continuity of communication with patients and their families is essential to establish clear expectations and sustain active participation; similarly, consistent medical and other clinical practices that prioritize rapid reconditioning, mitigation of in-hospital complications, and rapid return to baseline status are essential to achieve this goal. In the pre-procedure phase, patient teaching inclusive of the rationale for early mobilization and anticipated activity progression can help set expectations and promote active participation. Minimalist peri-procedure practices that promote early mobilization include the use of ultrasound-guided vascular access to reduce the risk of vascular injury, consideration of selective anticoagulation reversal to promote haemostasis, removal of the temporary pacemaker at the end of the procedure, and avoidance of invasive lines.18 In addition, a default strategy of local anaesthesia and light sedation contributes to a stable post-procedure haemodynamic status and predictable readiness for mobilization while reducing the risk of delirium.34,35 In the post-procedure phase, the availability of a standardized protocol and the dissemination of education focused on the risks of immobility and the safety of early mobilization are essential to achieve the adoption of best practices and nurses’ competency of post-TAVI ambulation.36 In the event of post-procedure complications, including delayed haemostasis or the onset of new conduction delay, continuity of medical care can effectively maintain the patient on the TAVI clinical pathway and reduce the risks of fragmented care and inappropriate bedrest.18
The right care in the right place
In our study, we found that the location of both the procedure and patients’ early recovery was associated with time to mobilization, with patients treated in the more intensive clinical areas (HOR and CICU) experiencing longer bedrest than patients who were cared for in the lower intensity clinical areas (CCL and cardiac telemetry unit). The pathway selection was incidental for some patients (e.g. timing and availability of resources), while driven by individual clinical factors for higher risk patients (e.g. selective use of the HOR for complex anatomical features and risk-stratified admission to critical care). While the study was not powered to parse these effects, we can speculate that differences may be driven by the combined effect of patient-level factors (i.e. non-modifiable) and organizational culture factors (i.e. modifiable). There is evidence that critical care nurses have inconsistent knowledge of the benefits of mobilizing patients and view mobilization as a low priority.36 In addition to time constraints, staffing levels, and competing unit demands,37 critical care nurses report uncertainty and ambiguity about their role with regard to the initiation of mobilization, the effect of existing cultural influences, and their hesitancy related to previous experience of complex mobilization.38 Education initiatives aimed at increasing nurses’ awareness of the negative effect of the historical bedrest culture—especially in critical care—promoting the uptake of contemporary evidence and practice changes, and empowering front line nurses to lead early ambulation may be effective in improving standardization of practice across clinical units used for TAVI.39
There is growing evidence that TAVI has become an increasingly predictable procedure, with a low incidence of post-procedure complications and safe next-day discharge; the avoidance of critical care for the recovery of most patients matches this contemporary evidence.40 The onset of COVID-19 and the competing demands for critical care resources further accelerated the transition to post-procedure care in the cardiac telemetry unit and leveraged the expertise of cardiovascular nurses.41 This shift in the trajectory of TAVI care may offer opportunities to extend the concept of minimalist TAVI to post-procedure care to improve outcomes and reduce health resources utilization without compromising patient safety.
Limitations
Although this study contributes new evidence to guide the timing of mobilization after TAVI, findings should be interpreted in light of several limitations. The study design was limited to an observational cohort in a single centre that utilizes multiple processes and clinical units to ensure access to care and where continuous quality improvement impacts rapid iteration changes in nursing practice. We recognize that a randomized clinical trial would provide stronger evidence. Most patients received a balloon-expandable device, with a low incidence of new conduction delays and the avoidance of transfer to critical care. In addition, regional differences in nurses’ clinical contexts may impact the generalizability of findings. Future research is needed to explore further optimization of mobilization and clinical risk stratification.
Conclusion
Nurse-led mobilization 4 h after TAVI is feasible for most patients who receive post-procedure care in cardiac telemetry or critical care units and is an important intervention to mitigate the risks associated with treating patients with heart valve disease. This study adds new evidence that suggests that TAVI is becoming a routine procedure amenable to standardized care and a streamlined approach to facilitate patients’ rapid return to baseline status and safe return home. In this new context, quality improvement that leverages contemporary evidence is essential to improving access to care and encouraging multidisciplinary teams to review, revise, and recalibrate best practices to promote excellent outcomes and appropriate health service delivery.
Author contributions
S.B.L.: conceptualization, investigation, methodology, supervision, validation, visualization, and writing (original draft). M.Y.: conceptualization, formal analysis, investigation, methodology, validation, visualization, and writing (review and editing). C.B.: conceptualization, investigation, funding acquisition, and project administration. B.B.: conceptualization, investigation, methodology, and writing (review and editing). J.P.: project administration, formal analysis, investigation, methodology, validation, visualization, and writing (review and editing). A.L.S.: investigation, project administration, validation, and writing (review and editing). E.D.: conceptualization, investigation, and writing (review and editing). M.A.: conceptualization, investigation, and writing (review and editing). D.M.: conceptualization, investigation, and writing (review and editing). H.A.: conceptualization, investigation, funding acquisition, and project administration. L.A.: conceptualization, investigation, funding acquisition, and project administration. E.T.: conceptualization, investigation, and writing (review and editing). D.A.W.: conceptualization, investigation, and writing (review and editing). J.S.: conceptualization, investigation, methodology, and writing (review and editing). J.G.W.: conceptualization, investigation, supervision, methodology, and writing (review and editing).
Acknowledgements
We are grateful for the significant contributions of cardiac telemetry, cardiac short-stay, and cardiac intensive care unit nurses for their generous support to collect study data.
Funding
The Providence Health Care (Vancouver, Canada) Practice-Based Research Challenge and the Office of Professional Practice supported this study. We gratefully acknowledge their financial support.
Data availability
Data are not available for further analyses due to the requirements of the UBC Research Ethics Board.
References
Author notes
Conflict of interest: S.B.L. has been a consultant for Edwards and Medtronic; J.S. has been a consultant for Edwards, Medtronic, and Boston Scientific and received research grants from Edwards and Medtronic; D.A.W. has received research grants from Abbott and Edwards; J.G.W. has been a consultant and/or received research support from Edwards, Abbott, Boston Scientific, and Vivitro Medical.




Comments