-
PDF
- Split View
-
Views
-
Cite
Cite
Miroslav Ferenc, Franz-Josef Neumann, Efficacy of primary PCI: the microvessel perspective, European Heart Journal Supplements, Volume 7, Issue suppl_I, October 2005, Pages I4–I9, https://doi.org/10.1093/eurheartj/sui061
Close - Share Icon Share
Abstract
The outcome of patients with acute myocardial infarction (AMI) is critically dependent on adequate reperfusion at the tissue level. Primary percutaneous coronary intervention (PCI) achieves full patency of the infarct-related vessel in >90% of the patients with AMI. Despite the restoration of large-vessel flow, tissue perfusion in area at risk frequently continues to be compromised. To optimize microvascular reperfusion and clinical outcomes, additional measures are needed. Thus far, mechanical approaches to improve distal perfusion, such as the use of distal protection devices, have not been shown beneficial. Among the pharmacological approaches, high-dose adenosine infusion holds promise but has to be proved by further clinical studies. Glycoprotein IIb/IIIa receptor blockade with abciximab has a documented efficacy in improving microvascular flow, contractile recovery, and patient survival after primary PCI in AMI. In addition to the inhibition of platelet aggregation, prevention of pro-inflammatory heterotypic platelet interactions may contribute to the beneficial effect of this drug.
Illusion of TIMI grade 3 flow
The treatment strategy for acute myocardial infarction (AMI) is rapidly evolving. Early and complete reperfusion has become the main goal of treatment. Restoration of unimpeded flow through the occluded coronary artery [i.e. thrombolysis in myocardial infarction (TIMI) grade 3 flow] is the prerequisite for survival benefit and recovery of contraction in the infarct area.1–3 Thus, until recently, TIMI grade 3 flow was considered to be predictive of optimal clinical benefit. However, it is becoming increasingly clear that restoration of large-vessel patency alone does not suffice to achieve the best outcome.
Despite the restoration of large-vessel flow, tissue perfusion in the area at risk frequently continues to be compromised, as shown by myocardial contrast echocardiography (MCE),4,5 flow velocity measurements,6–10 and assessment of TIMI frame counts11,12 or myocardial blush.13,14 Persistent microcirculatory impairment is associated with poor recovery of contractile function4–10 and adverse clinical outcomes.4,11–14 Thus, restoration of large-vessel patency does not mean complete perfusion recovery, and perfusion of the microvasculature is an additional prerequisite for obtaining optimal recovery.
Adjunctive therapies to improve microvascular reperfusion
The extent of microvascular perfusion deficits in the presence of TIMI grade 3 flow varies among patients4–14 and is a predictor of myocardial recovery4–10 and clinical outcomes.4,11–14 Therefore, the treatment for AMI should include attempts to correct microvascular perfusion as well as large-vessel perfusion.
Mechanical approaches
Since the early days of primary percutaneous coronary intervention (PCI), the fate of the occlusive thrombus material that is destructed by primary PCI has been a matter of concern. Systematic evaluation revealed angiographic evidence of distal embolization in 9–15% of the patients after primary PCI for AMI,15–17 but the true incidence of distal embolization may be considerably higher as suggested by autopsy studies18 and experience with distal protection devices.15–17 Distal embolization carries an increased risk of poor clinical outcomes; in a recent study, it was associated with an eight-fold increase in 5-year mortality.16
On the basis of these findings, distal embolization of plaque and thrombus material is considered to be a major cause for insufficient reperfusion, despite a fully patent infarct-related artery. Hence, it was hypothesized that distal protection devices that prevent embolization during primary PCI may improve distal perfusion.
This concept, however, could not be proved in randomized studies. The first of these trials was the Enhanced Myocardial Efficacy and Recovery by Aspiration of Liberalized Debris (EMERALD) study on distal balloon occlusion and aspiration.19 EMERALD study randomized 501 patients to distal protection or usual care. Visible debris that otherwise would have entered the distal circulation could be removed in 73% of the patients of the study group. Nevertheless, neither the primary endpoint ST-segment resolution nor any of the secondary endpoints including myocardial blush showed any significant benefit of distal protection when compared with usual care.
Concurrent results were obtained in the randomized controlled Protection Devices in PCI Treatment of Myocardial Infarction for Salvage of Endangered Myocardium (PROMISE) study.20 PROMISE study evaluated the effects of the filter wire on myocardial perfusion, as determined by the maximal blood flow velocity across the recanalized infarct-related artery. The study enrolled 200 patients with myocardial infarction (69% ST-elevation) within 48 h (median 6.9 h) after onset of pain. Adenosine-induced flow velocity in the recanalized infarct artery was 34±17 cm/s with the filter and 36±20 cm/s without (P=0.46). Infarct size measured by magnetic resonance imaging was 12±9% of the LV with the filter and 10±9% without (P=0.33). Hence, the filter did not improve reperfusion or reduce infarct size after primary PCI. The consistent results of EMERALD and PROMISE suggest that irrespective of the technology, distal protection does not improve reperfusion after primary PCI in myocardial infarction to a clinically detectable degree.
Pharmacological approaches: adenosine
Various pharmacological approaches to improve reperfusion have been tested. These included antioxidants to mitigate reperfusion-associated oxidative stress,21 rheological agents to improve blood viscosity,22,23 and anti-inflammatory agents.24,25 However, none of the clinical trials yielded convincing results. More recently, peri-interventional administration of adenosine has received much attention. In animal models of infarction, adenosine attenuates the no-reflow phenomenon and limits neutrophil activation and reperfusion injury.26,27 A clinical study of 54 patients suggested that adenosine as adjunct to primary percutaneous transluminal coronary angioplasty (PTCA) ameliorated flow and prevented the no-reflow phenomenon.28 However, the effect of adenosine was less convincing in the adequately powered clinical trial Acute Myocardial Infarction Study of Adenosine (AMISTAD II).29 In this study, 2118 patients with evolving anterior STEMI receiving thrombolysis or primary angioplasty were randomized to a 3-h infusion either of adenosine 50 or 70 µg/kg per minute or of placebo. Clinical outcomes in patients with STEMI undergoing reperfusion therapy were not significantly improved with adenosine. Nevertheless, infarct size was reduced with the 70-µg/kg per minute adenosine infusion, and this finding correlated with fewer adverse clinical events. The investigators of AMISTAD concluded that a larger study limited to the 70-µg/kg per minute dose was warranted.
Pharmacological approaches: GP IIb/IIIa receptor blockade with abciximab
Previous investigations on experimental models of myocardial infarction documented the pre-eminent role of platelets in limiting blood flow during reperfusion in AMI.30 By occlusive thrombus formation, distal embolization of small aggregates, and release of vasoconstrictive mediators, platelets interfere with both large-vessel patency and microvascular flow.30
The effect of abciximab on microvascular reperfusion was investigated in the first 200 patients enrolled in the ISAR-2 (Intracoronary Stenting and Antithrombotic Regimen-2) trial (Figure 1).9 After 2 weeks, patients in the abciximab arm showed a significantly improved recovery of peak-flow velocity (as measured using the Doppler wire) in the occluded coronary artery when compared with that of patients in the conventional treatment arm [mean increase in peak-flow velocity within 14 days (95% CI): 18.1 (13.6–22.6) cm/s vs. 10.4 (5.4–15.4) cm/s; P=0.024; Figure 1]. As quantitative coronary angiography did not reveal any difference in angiographic outcome between the two treatment groups, the beneficial effect of abciximab reflected improvement in microvascular function.
The recovery of microvascular function was correlated with a significant improvement in wall motion in patients receiving abciximab when compared with that of the control group. Thus, the improvement of wall motion index in the infarct region was significantly greater in patients assigned to abciximab than that in those on heparin alone [mean increase within 14 days: 0.44 (0.29–0.59) SD/chord vs. 0.15 (0.00–0.30) SD/chord; P=0.007; Figure 1].9 At 14-day follow-up, the abciximab-treatment group had a higher global LV ejection fraction than that of the heparin-treatment group [62 (59–65)% vs. 56 (53–59)%; P=0.003). This finding is consistent with other studies, illustrating a close relationship between the recovery of perfusion and contractile function in infarct region.4–10
ISAR-2 trial was the first study demonstrating that in AMI, abciximab has important effects beyond the maintenance of large-vessel patency. It improves the coronary perfusion recovery at the level of the distal vascular bed and concomitantly enhances the restoration of LV function in the infarct area. Several, more recent studies have confirmed the effect of abciximab on reperfusion of the distal vascular bed.
Mulumudi et al.31 used myocardial blush to study the effect of abciximab on myocardial microcirculation following PTCA and/or stenting for AMI. They found that 74% of patients receiving abciximab (n=27) had moderate-to-normal blush when compared with 45% of those in the control group (n=20). This effect was particularly striking in diabetics, almost two-thirds of diabetics receiving abciximab had normal blush when compared with no diabetics in the control group.
Petronio et al.32 investigated the effect of abciximab and adenosine on microvascular integrity and LV functional recovery in 47 AMI patients undergoing PTCA. When compared with controls receiving conventional therapy, MCE revealed an improvement in microvascular perfusion in the treatment groups, both immediately after PTCA and at 48 h. At 30 days, only abciximab remained superior to conventional treatment. Microcirculation recovery in the abciximab group translated into an improvement in wall motion at 3 months, indicating an improved recovery of LV function.
In a later study by Petronio et al.,33 the effect of abciximab on microvascular integrity and LV functional recovery was studied in 31 AMI patients (<6 h) treated with primary PTCA. Patients randomized to abciximab showed significant improvements in measures of myocardial reperfusion (corrected TIMI frame count, 23±4 vs. 30±9 frames; P<0.05). In addition, microvascular integrity, measured using MCE, was preserved both in the short-term [77 vs. 55% (P<0.01) after PTCA and 86 vs. 50% (P<0.005) after 48 h] and at 1-month follow-up (86 vs. 54%; P<0.001). Abciximab patients also showed better recovery of LV function, as indicated by an improved wall motion score index (1.4±0.3 vs. 1.5±0.2; P<0.05) and increased LV ejection fraction (53+7 vs. 48.5±5%; P<0.001). The authors concluded that in AMI patients treated with PTCA, abciximab can preserve microvascular integrity and thus assist LV function recovery.
Molecular mechanisms of abciximab effect
In contrast to other GP IIb/IIIa blockers, abciximab is non-specific and also blocks other integrins, such as Mac-1 (αM/β2)34 and the vitronectin receptor (αV/β3).35 In the absence of conclusive clinical studies on the use of the more specific GP IIb/IIIa blockers in the setting of AMI,36 the contribution of the non-GP IIb/IIIa-dependent receptor blockade to clinical effects of abciximab remains unclear. Nevertheless, experimental studies suggest that Mac-1 and vitronectin receptors play a key role during ischaemia and reperfusion (discussed subsequently).
Inhibition of thrombus formation
GP IIb/IIIa is a central receptor for homotypic and heterotypic platelet interactions.37 Most importantly, the final common pathway in platelet aggregation involves fibrinogen bridging between platelet GP IIb/IIIa receptors.37 By effectively blocking this receptor, abciximab prevents platelet aggregation.37 In the setting of AMI, abcixmab not only reduces ongoing platelet-thrombus formation at the culprit lesion and distal embolization of platelet microemboli but also other consequences of platelet aggregation, such as release of vasoconstrictive mediators38 and shedding of pro-thrombotic platelet-derived microparticles.39
Inhibition of heterotypic platelet-dependent cell interactions
During reperfusion, the interaction between platelets, leukocytes, and endothelial cells also has important effects on microvascular function. In this setting, P-selectin-mediated tethering of platelets to activated endothelium occurs and subsequent firm adhesion is caused by the interaction of GP IIb/IIIa-bound fibrinogen with endothelial vitronectin receptors and intercellular adhesion molecule-1 (ICAM-1).40,41 Through stimulation by CD40 ligand (CD40L) and platelet-bound interleukin-1 activity, binding of activated platelets to endothelial cells results in the expression of monocyte chemoattractant protein-1 and ICAM-1 via a nuclear factor-κB-dependent mechanism.40,41 Activated platelets thereby modulate chemotactic and adhesive properties of endothelial cells and direct leukocyte attachment; this has been demonstrated in cell culture studies.42
Platelets can also directly support leukocyte adhesion. Heterotypic interactions of endothelium-bound platelets appear to play an important role in the recruitment of leukocytes to the site of vascular injury.43 These involve the primary attachment of platelets to myeloid leukocytes by interactions between platelet P-selectin and P-selectin GP ligand-1 (PSGL-1) on the leukocyte surface.44–49 Heterotypic adhesion is stabilized by the binding of Mac-1 on the leukocyte to an unknown counter-receptor on the platelet.47,49,50 This interaction is a result of Mac-1 activation, which in turn is due to tyrosine phosphorylation and mitogen-activated protein kinase activation stimulated by PSGL-1 ligation.47,51 Leukocyte activation by stimulated platelets is further enhanced by CD40L-dependent signal transduction.52In vitro, the binding of activated platelets to myeloid leukocytes induces the expression of pro-inflammatory cytokines, oxidative burst, and an increased surface expression of tissue factor and Mac-1.53–56 Mac-1 is known to play a key role in leukocyte-dependent reperfusion injury.57–59
Abundant platelet–leukocyte aggregates have been found in the peripheral blood of patients with AMI.54 GP IIb/IIIa receptor blockade with abciximab in these patients reduces platelet–monocyte interaction by decreasing the mass of platelets attached to monocytes.42 Through this mechanism, abciximab decreases Mac-1 surface expression on circulating monocytes.60 This effect is complemented by the direct Mac-1-blocking properties of abciximab. In addition, cell culture experiments have shown that abciximab can prevent firm adhesion of platelets to activated endothelial cells by blocking both GP IIb/IIIa and the vitronectin receptors.34 The inhibitory effect of abciximab on heterotypic platelet interactions during ischaemia and reperfusion has been confirmed by experiments in isolated guinea pig hearts, in which abciximab improved the recovery of contractile function.61 These studies demonstrate the functional importance of heterotypic platelet interactions during ischaemia and reperfusion.
A recently published clinical study is consistent with a beneficial effect of the attenuation of pro-inflammatory platelet–endothelium interaction. Aymong et al.62 demonstrated that abciximab can attenuate endothelial dysfunction. Using the Doppler wire, the investigators studied acetylcholine-mediated coronary blood flow in 48 patients who underwent stenting either with abciximab or without abciximab and a control group of 31 patients who did not undergo coronary intervention or receive drug therapy. Acetylcholine-induced vasodilation was significantly impaired after stenting without abciximab (41% increase in flow vs. 70% in the control group), whereas in patients treated with abciximab during the intervention, the blood flow response to acetycholine was normal (83% increase in flow). The beneficial effect of abciximab on endothelium-dependent vasodilator responses may be interpreted as a consequence of the inhibition of platelet–endothelium interactions.
Clinical relevance of improved reperfusion by abciximab
Experimental and mechanistic clinical studies suggest a beneficial effect of abciximab on reperfusion after primary PCI in acute myocardial studies. Five larger randomized clinical studies addressed the clinical relevance of this effect. These studies included RAPPORT (ReoPro and Primary Percutaneous Transluminal Coronary Angioplasty Organization and Randomized Trial),63 ISAR-2 trial,64 ADMIRAL (Abciximab before Direct Angioplasty and Stenting in Myocardial Infarction Regarding Acute and Long-Term Follow-Up),65 CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications),66 and ACE (Abciximab Carbostent Evaluation).67,68 With respect to combined endpoint of death, recurrent myocardial infarction, and target vessel revascularization at 30 days, each of these trials showed a significant benefit of abciximab over control (Figure 2). At 6 months, all point estimates for the triple endpoint favour abciximab, but statistical significance was obtained in ADMIRAL and ACE only. Nevertheless, pooled analysis reveals a significant benefit of abciximab (Figure 2).
Concerning the hard endpoints, death, and non-fatal re-infarction, a meta-analysis on abciximab for primary PCI, which also included smaller studies, was published recently.69 This meta-analysis revealed a significant reduction by abciximab in the 30-day incidence of re-infarction when compared with that of control group (1.0 vs. 1.9%; P=0.03). Most importantly, when compared with the control group, abciximab was associated with a significant reduction in 30-day mortality (2.4 vs. 3.4%; P=0.047) and long-term (6–12 months) mortality (4.4 vs. 6.2%; P=0.01). Thus, peri-interventional administration of abciximab for primary PCI affords a sustained clinical benefit with improved survival.
Conflict of interest: none declared.
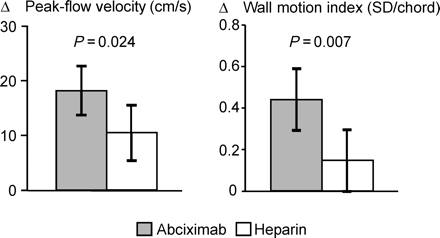
Figure 1 Increase between 14-day follow-up and initial post-interventional study in papaverine-induced peak-flow velocity in the recanalized and stented infarct-related artery (left panel) and in wall motion index within the infarct region (right panel). Columns represent the mean difference. Error bars indicate the 95% CI. Error bars not including zero indicate that the change between initial study and follow-up is statistically significant at the 0.05-level. P-values above the columns refer to the difference between the two treatment groups. (Reproduced with permission from Ref. 9)
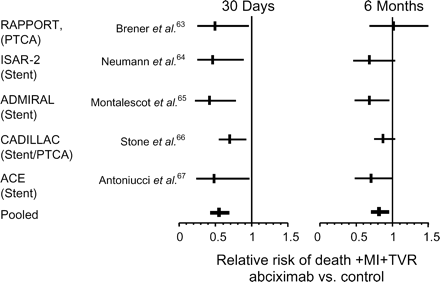
Figure 2 Meta-analysis of five major studies on abciximab during PCI for AMI. MI, recurrent myocardial infarction; TVR, target vessel revascularization.
References
Vogt A, von Essen R, Tebbe U et al. Impact of early perfusion status of the infarct-related artery on short-term mortality after thrombolysis for acute myocardial infarction: retrospective analysis of four German multicenter studies.
Simes RJ, Topol EJ, Holmes DR Jr et al. Link between the angiographic substudy and mortality outcomes in a large randomized trial of myocardial reperfusion. Importance of early and complete infarct artery reperfusion. GUSTO-I Investigators.
Anderson JL, Karagounis LA, Becker LC et al. TIMI perfusion grade 3 but not grade 2 results in improved outcome after thrombolysis for myocardial infarction. Ventriculographic, enzymatic, and electrocardiographic evidence from the TEAM-3 Study.
Ito H, Maruyama A, Iwakura K et al. Clinical implications of the ‘no reflow’ phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction.
Ito H, Okamura A, Iwakura K et al. Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction.
Wakatsuki T, Nakamura M, Tsunoda T et al. Coronary flow velocity immediately after primary coronary stenting as a predictor of ventricular wall motion recovery in acute myocardial infarction.
Mazur W, Bitar JN, Lechin M et al. Coronary flow reserve may predict myocardial recovery after myocardial infarction in patients with TIMI grade 3 flow.
Tsunoda T, Nakamura M, Wakatsuki T et al. The pattern of alteration in flow velocity in the recanalized artery is related to left ventricular recovery in patients with acute infarction and successful direct balloon angioplasty.
Neumann FJ, Blasini R, Schmitt C et al. Effect of glycoprotein IIb/IIIa receptor blockade on recovery of coronary flow and left ventricular function after the placement of coronary-artery stents in acute myocardial infarction.
Neumann FJ, Kosa I, Dickfeld T et al. Recovery of myocardial perfusion in acute myocardial infarction after successful balloon angioplasty and stent placement in the infarct-related coronary artery.
French JK, Hyde TA, Straznicky IT et al. Relationship between corrected TIMI frame counts at three weeks and late survival after myocardial infarction.
Gibson CM, Murphy SA, Rizzo MJ et al. Relationship between TIMI frame count and clinical outcomes after thrombolytic administration. Thrombolysis in Myocardial Infarction (TIMI) Study Group.
Gibson CM, Cannon CP, Murphy SA et al. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs.
van't Hof AW, Liem A, Suryapranata H et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group.
Giri S, Mitchel JF, Hirst JA et al. Synergy between intracoronary stenting and abciximab in improving angiographic and clinical outcomes of primary angioplasty in acute myocardial infarction.
Henriques JP, Zijlstra F, Ottervanger JP et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction.
Limbruno U, Micheli A, de Carlo M et al. Mechanical prevention of distal embolization during primary angioplasty: safety, feasibility, and impact on myocardial reperfusion.
Saber RS, Edwards WD, Bailey KR et al. Coronary embolization after balloon angioplasty or thrombolytic therapy: an autopsy study of 32 cases.
Stone GW, Webb J, Cox DA et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial.
Gick M, Jander N, Bestehorn HP et al. Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation.
EMIPFR Group. Effect of 48-hour intravenous trimetazidine on short- and long-term outcomes of patients with acute myocardial infarction, with and without thrombolytic therapy. A double-blind, placebo-controlled randomized trial.
CORE Investigators. Effects of Rheoth Rx on mortality, morbidity, left ventricular function, and infarct size in patients with acute myocardial infarction.
Wall TC, Califf RM, Blankenship J et al. Intravenous fluosol in the treatment of acute myocardial infarction. Results of thrombolysis and angioplasty in myocardial infarction 9 trial.
Faxon DP, Gibbons RJ, Chronos NA et al. The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: the results of the HALT-MI study.
Baran KW, Nguyen M, McKendall GR et al. Double-blind randomized trial of an anti-CD18 antibody in conjunction with recombinant tissue plasminogen activator for acute myocardial infarction.
Kaminski PM, Proctor KG. Attenuation of no-reflow phenomenon, neutrophil activation, and reperfusion injury in intestinal microcirculation by topical adenosine.
Babbitt DG, Virmani R, Forman MB. Intracoronary adenosine administered after reperfusion limits vascular injury after prolonged ischemia in the canine model.
Marzilli M, Orsini E, Marraccini P et al. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction.
Ross AM, Gibbons RJ, Stone GW et al. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II).
Topol EJ. Toward a new frontier in myocardial reperfusion therapy. Emerging platelet preeminence.
Mulumudi MS, Potluri SP, White CJ. Role of abciximab in the preservation of myocardial microcirculation during mechanical reperfusion for acute ST segment elevation myocardial infarction. (Abstract).
Petronio AS, Musumeci G, Nardi C et al. Microcirculation recovery after primary coronary angioplasty in patients with acute myocardial infarction treated with abciximab or intracoronary adenosine. (Abstract).
Petronio AS, Rovai D, Musumeci G et al. Effects of abciximab on microvascular integrity and left ventricular functional recovery in patients with acute infarction treated by primary coronary angioplasty.
Simon DI, Xu H, Ortlepp S et al. 7E3 monoclonal antibody directed against the platelet glycoprotein IIb/IIIa cross-reacts with the leukocyte integrin Mac-1 and blocks adhesion to fibrinogen and ICAM-1.
Tam SH, Sassoli PM, Jordan RE et al. Abciximab (ReoPro, chimeric 7E3 Fab) demonstrates equivalent affinity and functional blockade of glycoprotein IIb/IIIa and alpha(v)beta3 integrins.
Büttner HJ, Neumann FJ. Tirofiban for catheter intervention in acute myocardial infarction?
Coller BS. GPIIb/IIIa antagonists: pathophysiologic and therapeutic insights from studies of c7E3 Fab.
Willerson JT, Yao SK, McNatt J et al. Frequency and severity of cyclic flow alternations and platelet aggregation predict the severity of neointimal proliferation following experimental coronary stenosis and endothelial injury.
Morel O, Hugel B, Jesel L et al. Circulating procoagulant microparticles and soluble GPV in myocardial infarction treated by primary percutaneous transluminal coronary angioplasty. A possible role for GPIIb–IIIa antagonists.
Massberg S, Enders G, Leiderer R et al. Platelet-endothelial cell interactions during ischemia/reperfusion: the role of P-selectin.
Gawaz M, Neumann FJ, Dickfeld T et al. Vitronectin receptor (αvβ3) mediates platelet adhesion to the luminal aspect of endothelial cells. Implications for reperfusion in acute myocardial infarction.
Kuijper PH, Gallardo Torres HI, van der Linden JA et al. Platelet-dependent primary hemostasis promotes selectin- and integrin-mediated neutrophil adhesion to damaged endothelium under flow conditions.
Kirchhofer D, Riederer MA, Baumgartner HR. Specific accumulation of circulating monocytes and polymorphonuclear leukocytes on platelet thrombi in a vascular injury model.
Hamburger S, McEver RP. GMP-140 mediates adhesion of stimulated platelets to neutrophils.
Rinder HM, Bonan JL, Rinder CS et al. Activated and unactivated platelet adhesion to monocytes and neutrophils.
Rinder HM, Bonan JL, Rinder CS et al. Dynamics of leukocyte–platelet adhesion in whole blood.
Evangelista VE, Manarini S, Rotondo S et al. Platelet/polymorphonuclear leukocyte interaction in dynamic conditions: evidence of adhesion cascade and cross talk between P-selectin and the ß2 integrin CD11b/CD18.
Diacovo TG, Roth SJ, Buccola JM et al. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the ß2-integrin CD11b/CD18.
Konstantopoulos K, Neelamegham S, Burns AR et al. Venous levels of shear support neutrophil–platelet adhesion and neutrophil aggregation in blood via P-selectin and ß2-integrin.
Sheikh S, Nash GB. Continuous activation and deactivation of integrin CD11b/CD18 during de novo expression enables rolling neutrophils to immobilize on platelets.
Hidari KI, Weyrich AS, Zimmerman GA et al. Engagement of P-selectin glycoprotein ligand-1 enhances tyrosine phosphorylation and activates mitogen-activated protein kinases in human neutrophils.
Henn V, Slupsky JR, Grafe M et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells.
Nagata K, Tsuji T, Todoroki N et al. Activated platelets induce superoxide anion release by monocytes and neutrophils through P-selectin (CD62).
Neumann FJ, Marx N, Gawaz M et al. Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets.
Ott I, Neumann FJ, Gawaz M et al. Increased neutrophil–platelet interaction in patients with unstable angina.
Barry OP, Pratico D, Savani RC et al. Modulation of monocyte–endothelial cell interactions by platelet microparticles.
Nolte D, Hecht R, Schmid P et al. Role of Mac-1 and ICAM-1 in ischemia–reperfusion injury in a microcirculation model of BALB/C mice.
Neumann FJ, Ott I, Gawaz M et al. Cardiac release of cytokines and inflammatory responses in acute myocardial infarction.
Ma XL, Tsao PS, Lefer AM. Antibody to CD-18 exerts endothelial and cardiac protective effects in myocardial ischemia and reperfusion.
Neumann FJ, Zohlnhöfer D, Fakhoury L et al. Effect of glycoprotein IIb/IIIa receptor blockade on platelet–leukocyte interaction and surface expression of the leukocyte integrin Mac-1 in acute myocardial infarction.
Kupatt C, Habazettl H, Hanusch P et al. c7E3Fab reduces postischemic leukocyte–thrombocyte interaction mediated by fibrinogen. Implications for myocardial reperfusion injury.
Aymong ED, Curtis MJ, Youssef M et al. Abciximab attenuates coronary microvascular endothelial dysfunction after coronary stenting.
Brener SJ, Barr LA, Burchenal JE et al. Randomized, placebo-controlled trial of platelet glycoprotein IIb/IIIa blockade with primary angioplasty for acute myocardial infarction.
Neumann FJ, Kastrati A, Schmitt C et al. Effect of glycoprotein IIb/IIIa receptor blockade with abciximab on clinical and angiographic restenosis rate after the placement of coronary stents following acute myocardial infarction.
Montalescot G, Barragan P, Wittenberg O et al. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction.
Stone GW, Grines CL, Cox DA et al. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction.
Antoniucci D, Rodriguez A, Hempel A et al. A randomized trial comparing primary infarct artery stenting with or without abciximab in acute myocardial infarction.
Antoniucci D, Migliorini A, Parodi G et al. Abciximab-supported infarct artery stent implantation for acute myocardial infarction and long-term survival: a prospective, multicenter, randomized trial comparing infarct artery stenting plus abciximab with stenting alone.
- myocardial infarction, acute
- adenosine
- abciximab
- percutaneous coronary intervention
- blood platelets
- platelet glycoprotein gpiib-iiia complex
- reperfusion therapy
- infarction
- muscle contraction
- perfusion
- pharmacology
- platelet aggregation
- treatment outcome
- tissue perfusion
- distal embolic protection
- microvessels
- infusion procedures
- prevention
- large blood vessels
- fluid flow



