-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Rebecchi, Ermenegildo De Ruvo, Antonella Sette, Domenico Grieco, Lucia De Luca, Stefano Strano, Marco Tomaino, Domenico Giamundo, Stefano Sasso, Chiara Carabotta, Pietro Desimone, Alessandro Fagagnini, Cinzia Crescenzi, Annamaria Martino, Germana Panattoni, Fabiana Romeo, Marianna Sgueglia, Francesco Barillà, Michele Brignole, Leonardo Calò, Endocardial gaglionated plexi ablation in different vagally-mediated clinical settings: From cardioneuroablation to cardio-neuromodulation, European Heart Journal Supplements, Volume 27, Issue Supplement_1, February 2025, Pages i171–i176, https://doi.org/10.1093/eurheartjsupp/suae109
Close - Share Icon Share
Abstract
Cardioneuroablation (CNA) is now recognized as a safe and effective method in patients with cardioinhibitory neurocardiogenic syncope (CNCS), especially in young patients in order to avoid or prolong, as much as possible, the timing of definitive cardiac pacing. Several investigations have shown beneficial and very satisfactory results with a standard non-extensive endocardial ablation, aimed at identifying high-amplitude fragmented signals in the right and left atria. Despite this, the current scientific debate is focused about a proposal on an ablative method, even more individualized than CNA (at least as a first approach), considering that a standardized approach, especially in the left atrium, could expose CNCS patients with a good prognosis to an excessive risk of complications. These findings, moving from the concept of CNA to a new concept of ‘cardioneuromodulation’, opened a new era, aimed at a non-extensive and individualized treatment of different clinical CNCS scenarios or vagally-mediated atrioventricular block or sinus-atrial node dysfunction.
Introduction
Cardioneuroablation (CNA), despite heterogeneous aspects in terms of approach and medium- to long-term follow-up results, is now recognized as an effective procedure of ganglionated plexi (GPs) ablation for the treatment of traumatic cardioinhibitory neurocardiogenic syncope (CNCS) in young patients, especially with the aim of postponing as much as possible the time of pacemaker implantation.1,2
Generally, CNCS, characterized by bradycardia, systole, or atrioventricular (AV) block secondary to predominant parasympathetic hyperactivity, has a good prognosis in the absence of structural heart disease.3 However, the quality of life is severely compromised, particularly in the setting of frequent syncopal events.3 Current guidelines recommend cardiac pacing in patients above 40 years of age with spontaneous documented asystolic pauses (due to sinus arrest) of more than 3 and 6 s in symptomatic and asymptomatic patients, AV block or a combination of both.4 The indication for pacemaker implantation in a young patient should be considered very carefully, considering the need of multiple pacemaker replacements during the time and the associated risk of the device infection. The role of the CNA fits very well into this clinical scenario.
Moreover, CNCS is not the one and only application of CNA. In fact, several examples of vagal responses during pulmonary vein isolation have been described in the scientific literature,5 as well as experience with endocardial vagal denervation (often extensive) specifically aimed at treating patients with vagally-mediated atrial fibrillation (AF).6,7
At this point, it is legitimate to ask whether an extensive vagal denervation ablation method, which could be considered necessary (or in any way uncritical) in patients with vagally-mediated AF, is correct to be applied also in CNCS patients, which in fact have a good prognosis. In fact, in these latter patients, very satisfactory results have also been obtained with non-extensive endocardial ablation aimed at identifying high-amplitude fragmented signals in the right atrium (RA) and left atrium (LA). These findings opened a new era, moving from the concept of vagal denervation to the more appropriate concept of CNA8 and the newly available concept of ‘cardioneuromodulation’, aimed at a non-extensive and individualized treatment of different clinical CNCS scenarios or vagally-mediated AV block or sinus-atrial node (SAN) dysfunction.
Therefore, the purpose of this review is to present a current critical analysis of the literature regarding the role of CNA in different clinical settings.
Anatomical and pathophysiological substrate of cardiac ganglionated plexus ablation
Previous anatomical and physiological studies in both animal and human models have well characterized the topographical and pathophysiological role of the cardiac GP. Among the pioneers of studies on the topographic anatomy of GPs, Armour et al.9 identified five main atrial GPs (Figure 1, Panel A): (i) the superior right atrial GP [located on the posterior superior surface of the RA adjacent to the junction of the superior vena cava (SVC) and RA]; (ii) the superior left atrial GP (ganglia identified on the posterior surface of the LA between the PVs); (iii) the posterior right atrial GP (on the posterior surface of the RA adjacent to the interatrial groove); (iv) the posteromedial left atrial GP (on the posterior medial surface of the LA); and finally, (v) the postero-lateral left atrial GP (on the posterior lateral surface of the left atrial base on the atrial side of the AV groove).
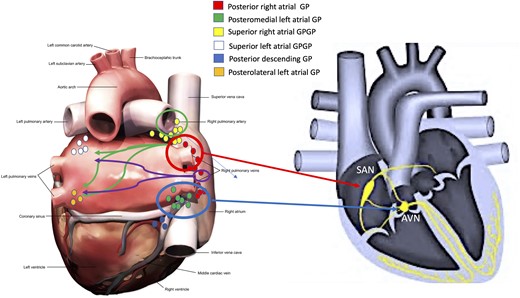
Location of the main atrial ganglionated plexi (A) according to Armour’s anatomical studies9 and (B) influence on the function of the atrioventricular node (see arrows) and sino-atrial node (see arrows). Also, neural interconnections between the right and left atrial main ganglionated plexi were noted (see arrows directed from right to left atrium). AVN, atrioventricular node; GP, ganglionated plexi; SAN, sino-atrial node.
Hou et al.10 and Pachon et al.8 are among the first to have explained the physiological and pathophysiological aspects of GP functions. Hou et al. studied the effect of vagal stimulation on SAN and atrioventricular node (AVN) function before and after sequential ablation of the superior left GP (SLGP, near the left superior pulmonary vein), anterior right GP (ARGP, near the SAN) and inferior right GP [IRGP, at the junction of the inferior vena cava (IVC) and the atria], confirmed that the large epicardial GPs can be considered as ‘integration centres’ that integrate autonomic innervation between the extrinsic and intrinsic cardiac autonomic nervous systems (Figure 2, Panel A). Indeed, the IRGP is considered the integration centre for the extrinsic autonomic nervous system to innervate the AVN (Figure 1, Panel B), while the ARGP is considered the integration centre for both the right and left vago sympathetic trunks to modulate SAN function (Figure 1, Panel B).
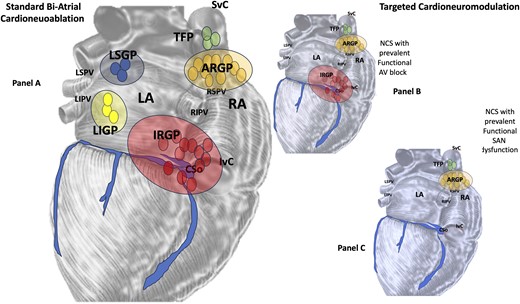
(A) Bi-atrial standard cardioneuroablation: an endocardial vagal denervation methodology based on the identification (by Fast Fourier Transformation) of fragmented signals in the (left and right atrium, respectively), markers of the neuro-myocardial interface (fibrillar myocardium), where parasympathetic fibres are located in the context of the atrial wall compact myocardium. (B) and (C) ‘Cardioneuromodulation’ could be more appropriate when considering an ablative non-extensive and personalized procedure according to different clinical settings. LA, left atrium; RA, right atrium; SVC, superior vena cava; IVC, inferior vena cava; RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; TFP, third fat pad; IRGP, inferior right ganglionated plexi; ARGP, anterior right ganglionated plexi; LSGP, left superior ganglionated plexi; LIGP, left inferior ganglionated plexi.
Pachon et al.,8,11 introducing the concept of ‘CNA’, first characterized as an endocardial vagal denervation methodology based on the identification (by Fast Fourier Transformation) of fragmented signals in the LA and RA, markers of the neuro-myocardial interface (fibrillar myocardium), where parasympathetic fibres are located in the context of the atrial wall compact myocardium. This pattern was found mainly in the areas of the SAN and the AVN and in the endocardial regions close to the three main atrial GPs [ganglion A, located between the SVC and the aortic root just above the right superior pulmonary vein (RSPV), ganglion B, located between the RSPV and the RA, and finally ganglion C, located between the IVC and the RA and LA].
Cardioneuroablation for cardioinhibitory neurocardiogenic syncope
Bi-atrial and left cardioneuroablation
Several studies have shown a satisfactory outcome of bi-atrial or left atrial ablation (Figure 1, Panel B) in patients with CNCS, although there is heterogeneity of approach. Pachon et al.11 showed in 43 patients with CNCS only three cases of spontaneous syncope at a mean follow-up of 45.1 ± 22 months after bi-atrial ablation. Furthermore, a long-term atropine test after ablation (21.7 ± 11 months) was negative in 76.7%, partially positive in 16.3% and normal in 6.9% of patients, reflecting long-term vagal denervation. A few years later, Pachon et al.12 demonstrated a higher success rate of CNA with denervation confirmation by extra-cardiac vagal stimulation, in terms of reduced risk of syncope recurrence, also compared with procedures without vagal denervation confirmation.13 Aksu et al.14 undertook CNA in 51 CNCS patients using a combination of high-frequency stimulation and spectral analysis to identify bi-atrial GP sites. During a mean follow-up of 11 months, 48 patients were free of syncopal episodes and the head-up tilt test was negative in 86.2% of the treated patients. Hu et al.15 reported good procedural success in 115 patients undergoing left atrial ARGP ablation (90% freedom from syncopal episodes). Piotrowsky et al.16 demonstrated the efficacy of bi-atrial CNA in 48 patients randomized to bi-atrial CNA (n = 24) vs. control, with a significant improvement in the quality of life.
Right atrial cardioneuroablation only
Anatomical and pathophysiological studies may support the theory of limiting ablation to the RA (at least as a first approach, Figure 2, Panel B). First, anatomical studies8 have shown that at least 50% of the atrial GPs are located at the level of the posterior right atrial wall adjacent to the interatrial groove (the so-called right atrial GPs). Among these, the main GPs considered as anatomical targets of the CNA procedure are the following:
The ‘third fat pad’ (TFP) (or the superior right atrial GP) located on the posterior surface of the RA, adjacent to the junction of the SVC and the aorta. The ‘TFP’ represents the junction point for vagal input to the GP before it innervates the atria,1,7,17,18
The ARGP and IRGP are considered integration centres for autonomic innervation of the SAN and AVN, respectively,10
The presence of multiple bi-atrial neural connections that may favour modulation from the right atrial GPs to the left atrial GPs.7 Calò et al.18 showed a good outcome of RA GP ablation at a mean long-term follow-up of 34.1 ± 6.1 months. Debruyne et al.19 archived a 95% reduction in syncope burden after RA CNA in 20 CNCS patients at 6 months follow-up.
Cardioneuroablation for atrioventricular block and sino-atrial node dysfunction
Several investigations have performed a focus on the use of CNA also in patients affected by vagal related AV block or functional AV block8,20–22 The presence of symptomatic AV block, the absence of structural cardiopathy, the functional nature of AV block, patients aged ≤60 years, and a complete resolution of AV block by atropine challenge test, could be considered elements to candidate patients for CNA.20 Aksu et al.,21 who analysed 231 patients with symptomatic AV block, performed CNA in 31 selected patients with functional AV block. Procedural success, defined as acute reversal of AV block and complete abolition of atropine response, was achieved in 30 (96.7%) cases, while the remaining patient received a pacemaker. At a mean follow-up of 19.3 ± 15 months, recurrence of AV block was observed in only 2 (6.7%) of the 30 procedurally successful cases (Figure 1, Panels A and B).
Functional SAN dysfunction could also be considered as a field of application for CNA. The potential candidates can be selected considering the following parameters, as shown by Aksu et al.20: sinus bradycardia or symptomatic sinus arrest, absence of structural cardiopathy, exclusion of intrinsic sinus node dysfunction with positive atropine response (a sinus rate increase of ≥25% or a sinus rate ≥90 b.p.m. with 0.04 mg/kg intravenous atropine sulphate); and age ≤60 years. Zhao et al.22 observed good success after CNA in 11 patients with functional sinus node dysfunction. In this study, patients with a corrected sinus node recovery time of more than 525 ms were excluded from the study cohort, although the results of the Aksu study showed excellent success in this group.20 Finally, Debruyne et al.19 first showed that non-extensive ‘cardioneuromodulation’, using a right-sided and computed tomography guided procedure, is safe, fast and highly reproducible in preventing inappropriate functional sinus bradycardia and syncope recurrence. This approach is aimed to create a partial and adequate vagolysis of the SAN of ARGP, without adopting an extensive bi-atrial approach, with associated risk of complications.
What experience has taught us: critical issues and future perspectives
In recent years, we have seen different approaches to performing CNA (Figure 2, Panel A): left atrial, bi-atrial extensive, right atrial only, etc. Several meta-analysis have shown the greater effectiveness of a LA and bi-atrial approach when compared with the single RA approach.23,24 However, these comparison studies, showed different ablative approaches (mapping fractionated electrograms, spectral method or high-frequency stimulation, purely anatomically guided method, etc.). This aspect and the absence of randomized trials on large populations are important limitations in celebrating one ablative approach over another.
Moreover, periprocedural and short terms complications of a left atrial CNA ablation should potentially be the same of those observed in AF ablation. These are mostly secondary to transseptal puncture and left atrial manipulation of mapping and ablation catheters in the LA: cardiac perforation with haemopericardium/tamponade, large vein stenosis, injury of the phrenic nerve or coronary arteries, oesophageal damage, bleeding, SAN artery damage described after bi-atrial CNA, etc.25 The right atrial CNA ablation is not without complications (phrenic nerve paralysis, SAN injury, and cardiac perforation, etc.) but clearly less serious than left- or bi-atrial ablation.
But the real question is not so much the overall risk of CNA complications, but whether to undergo to this unjustified risk CNCS patients with a good clinical prognosis.
Our group18 also proposed a hybrid ablative approach for CNCS patients, based on the experience with GP ablation for vagal AF7: CNA combined with an approach aimed at identifying the main GPs in the RA, based on anatomical knowledge. The rationale for an ‘extensive’ anatomical ablation approach was based on the imperfect knowledge of the exact anatomical borders of the GP clusters. Indeed, in our study, patients with CNCS recurrence after ablation showed less extensive RF lesions, especially at the level of IRGP, ARGP and ‘TFP’ sites. At the same time, however, if an extended anatomical approach can be considered effective in patients with vagal AF, there is no certainty that this approach may be excessive in young patients with CNCS. In fact, although the learning curve for CNA has certainly improved over time, it must be considered that the periprocedural complications, especially with a left atrial approach, are potentially the same as with AF ablation. The definition of CNA is already indicative of a non-extensive ablative approach aimed at vagal denervation in anatomic areas of GP localization, based on the identification of fragmented potentials indicative of the presence of endocardial neural fibres.
The concept of ‘cardioneuromodulation’ (Figure 2, Panels B and C), may be more appropriate considering a non-extensive and personalized ablative procedure, according to different clinical settings. For example, in the case of CNCS treatment (with evidence of functional AV block), we believe that, at least initially, an approach (whether in the right only or in the left) targeting only ARGP and IRGP ablation may be sufficient. Similarly, in the case of patients with CNCS and signs of functional SAN dysfunction, a personalized initial approach aimed at ARGP ablation could be very beneficial, as previously described by Debruyne et al.19
Therefore, there are some take-home messages to be highlighted:
The CNA efficacy in patients with vasovagal syncope and in functional forms of AV block and SAN dysfunction is undeniable;
However, the heterogeneity of the different approaches proposed and the lack of randomized trial in a large population represent some of the critical points of CNA26;
An extensive CNA approach could be excessive and not without complications in relation to the good prognosis of the diseases described above. It should not be forgotten that some investigations have shown an intermediate long-term risk of atrial and ventricular arrhythmias due to excessive sympathetic-vagal imbalance, increased heart rate and tissue reinnervation.27
Finally, at this time, it is reasonable to propose non-extensive procedures (so-called ‘cardioneuromodulation’) tailored to the different clinical settings of patients affected by autonomic nervous system imbalance.1,15,19
In the rare cases of young CNCS patients with a necessary indication for cardiac pacing (due to CNA failure), despite the effectiveness of algorithms such as ‘closer loop stimulation’28 in transvenous pacemakers, the future scenario could be represented by leadless pacemaker implant. In fact, several investigations are showing an equivalent efficacy and safety of a single-chamber leadless pacing in reducing syncopal events when compared with dual-chamber transvenous pacemaker.29
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
Author notes
M.R. and E.D.R. are first authors of the article.
Conflict of interest: none declared.



