-
PDF
- Split View
-
Views
-
Cite
Cite
Gennaro Cice, Leonardo Calò, Can we slow down the decline in renal function?, European Heart Journal Supplements, Volume 27, Issue Supplement_1, February 2025, Pages i149–i153, https://doi.org/10.1093/eurheartjsupp/suae123
Close - Share Icon Share
Abstract
The ‘chronic kidney disease’ (CKD) definition that best outlines the complex syndrome commonly called ‘kidney failure’ has become a problem of World Public Health due to its incidence and prevalence and due to exponentially increasing costs in every part of the world. The progressive reduction in the glomerular filtration rate, as known, goes hand in hand with an increase in cardiovascular risk understood as fatal and non-fatal heart attack, stroke, heart failure, and mortality. Therefore, every effort must aim at preventing or slowing down the decline in renal function in order to reduce not only critical renal events (the need for dialysis or transplantation among the most dreadful) but also the incidence of cardiovascular events. Since the disease is asymptomatic for a long time (often its detection is occasional and done with guilty delay), it is clearly important to make a correct and early evaluation of renal function with appropriate methods. Furthermore, it is crucial to make an aetiological diagnosis, when it is possible, of CKD because this will allow for the most targeted therapy possible. For a long time, an effective approach for the majority of people with CKD could only count on strict control of the diabetic disease and its complications, optimization of high blood pressure values, and the mandatory use of drugs blocking the renin–angiotensin–aldosterone system, particularly in the presence of albuminuria. Over time, this strategy proved to be only partially effective and the majority of patients nonetheless showed a progressive worsening of renal function. Only recently have we had access to two classes of innovative drugs such as glyphozines and incretins which have established themselves on the therapeutic scene because they have shown to be able to slow down the progression of CKD, first in patients with type 2 diabetes and subsequently in patients with CKD whether or not they have diabetes. Unexpectedly and convincingly, they have also been shown to significantly impact cardiovascular prognosis. From initially antidiabetic drugs, their effectiveness has forced the medical iconography to enrich itself with a new therapeutic niche by rightly speaking of ‘cardio-nephro-metabolic’ drugs.
‘Chronic kidney disease’ (CKD) has long been a problem of World Public Health and shows a continuously increasing incidence and prevalence with disturbing epidemiological data.1 In fact, it is estimated that more than 730 million individuals in the world suffer from it and that the disease resulted in 2019 alone (latest reliable data) in more than one million six hundred thousand deaths and thirty-seven million individuals with disabilities.2 In Italy, recent data estimate that about 10% of the population, therefore about 6 million, is affected by renal dysfunction.
The term CKD (which we should all use) correctly defines everything we have called ‘always’ renal insufficiency (Figure 1). Definition and staging should be used to describe CKD across the spectrum and along the entire disease trajectory.
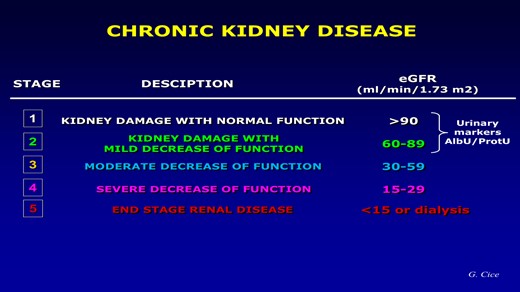
Even if everyone’s attention is understandably focused on that ‘cut-off’ of 60 mL/min below which, by convention, we can officially speak of kidney disease, it is of fundamental importance to remember that the utmost attention must be aimed at those individuals, not yet patients, who, despite the absence of renal structural alterations, present only microalbuminuria defined as a urinary excretion of albumin between 30 and 300 mg/day or as an albuminuria/creatinuria ratio on spot urine (UACR) between 30 and 300 mg/g. This finding, far from being a simple indicator of renal damage as long believed, has proved to be a formidable marker of endothelial dysfunction and systemic organ damage, capable of impacting the cardiovascular prognosis even more than on the progression of CKD, regardless of the extent of filtrate decline, even in the general population.3 All this to confirm, if ever there was still a need, of how much CKD is able to increase cardiovascular risk (fatal or non-fatal heart attack, stroke, disability-related, and heart failure).
Since most patients with uraemia die of cardiovascular causes even before going on dialysis, it will not be enough to treat these patients but it will be necessary to ‘take care of them’ by preventing and/or slowing down the progression of CKD in order to also reduce the incidence of new cardiovascular events.
Making a correct and early assessment of renal dysfunction is the first step to impact on the prognosis. It is useful to remember that a precise evaluation of chronic renal function can be obtained by evaluating its clearance with exogenous markers such as inulin or other less used substances such as chromium ethylenediaminetetraacetate (Cr-EDTA) or iothalamate. These substances have in common the ability to be filtered by the glomeruli but not reabsorbed or secreted by the tubules, thus giving an exact measure of the filtrate. However, these are long and expensive investigations and above all they do not respond to the clinical need for repeated checks and rapid responses. All of this has favoured the use of creatininaemia for a long time (less frequently of cystatinaemia). It retains a certain value especially in the context of screening tests of populations but does not express the real value of the filtrate due to its prolonged half-life (about 7–9 h) and to have an exponentially inverse relationship with the filtrate. Nephrological research then provided us with the ‘equations derived from creatininaemia’ whose acronyms (Cockcroft-Gault, MDRD Study Equation, CKD-EPI just to mention the most used) quickly entered into common use.4–8 However, it is useful to remember that the aforementioned formulas are not exact measurements but estimates of the filtrate!
Clearly making an aetiological diagnosis of CKD where possible will allow a case-specific therapy which, in particular cases, also makes use of immunosuppressive therapy.
In general, the attempt to slow down the decline in renal function for a long time could only take advantage of the control of hypertension and diabetes, on the inhibition of the renin–angiotensin–aldosterone system (RAAS), on changes in diet and lifestyle, and, as the disease progressed, on the correction of the metabolic acidosis and on the strict control of the calcium–phosphorus balance. Careful monitoring was added of all those nephrotoxic substances (classic example antineoplastic drugs) that one might eventually be forced to take. Given the complexity and vastness of the topic, it will be advisable to concentrate, albeit in a synthetic way, on those conditions that the clinician most frequently encounters in his daily work.
Inhibition of the renin–angiotensin–aldosterone system
Multiple trials have shown that therapy with angiotensin-converting enzyme-inhibitors (ACE-I) or Sartans (ARBs) is able to slow down the decline in renal function, especially in the presence of albuminuria.
In the REIN trial, patients with CKD randomized to ramipril vs. placebo showed a highly significantly reduced decline in renal function in the ACE-I arm, especially if proteinuria >3 g/dL was present.9
In the RENAAL study, patients with type 2 diabetes with CKD randomized to losartan had a 16% risk reduction in significant renal endpoints such as doubling of creatinine, need for dialysis, or death when compared with placebo.10 And as far as sartans are concerned, similar results were obtained, for example, in the IDNT trial where irbesartan was compared with amlodipine. Finally, in the AASK trial, ramipril use was independently associated with a −22 and −38% composite risk (filtrate decline >50% from baseline, need for dialysis, or death) when compared with metoprolol and amlodipine.7
The combined use of an ACE-I with a sartan, although still widely used in the nephrological field due to their synergistic action on proteinuria, is not supported by the current literature in diabetics with CKD. The NEPHRON-D study, which enrolled patients with type 2 diabetes with CKD randomized to losartan + lisinopril or losartan alone, was terminated due to an excess of adverse events (hyperkalaemia and episodes of acute renal failure) in the arm treated with the comparative combination with the monotherapy group.10,11
The importance of reducing the daily sodium intake in the diet is also reaffirmed because this amplifies the nephroprotective effect of the RAAS antagonist drugs. An important meta-analysis of 11 randomized controlled trials shows that a low-sodium diet per se reduces urinary albumin excretion by 32%. The reduction in albuminuria becomes even more significant if it is accompanied by an anti-RAAS therapy (−41 vs. −17%, respectively), suggesting a synergistic effect between a low-sodium diet and ACE-I or ARB therapy.12 Therefore, all patients taking RAAS inhibitors for the treatment of albuminuria should be encouraged to follow a diet with reduced sodium intake (−3 g/day).
Finally, the use of mineralocorticoid antagonist (MRA) in patients intolerant to ACE-I and ARB can be considered. This is because a recent meta-analysis of 31 randomized controlled trials, aimed at evaluating the efficacy and tolerability of different MRAs (spironolactone, eplerenone, canrenone, and finerenone) in reducing albuminuria compared with placebo or active drug confirmed the efficacy of these drugs in reducing the albuminuria vs. placebo but showed no superior effect when compared with ACE-I or ARB in the face of highly significant risk of hyperkalaemia.13 More recently, much discussion has been generated by the data from the FIDELIO-DKD study. Although the reduction in albuminuria is not universally accepted as a surrogate endpoint of the ‘need for dialysis’, finerenone has been shown to reduce the composite risk of renal events by 18% in patients with CKD with type 2 diabetes receiving ACE-I or ARB therapy such as filtrate decline, need for dialysis, and CKD-related deaths when compared with placebo.13
Ultimately, MRAs reduce albuminuria and slow down the progression of CKD but involve an increased risk of hyperkalaemia which requires careful control in daily practice.
Glycaemic control
The K-DIGO diabetes guidelines in CKD recommend individualizing the glycated haemoglobin (HbA1c) level to be achieved on the basis of the severity of the renal disease, the comorbidities, and the individual risk of hypoglycaemia.14 This is because if commonly used drugs such as insulin, sulfonylureas, and glinides are often responsible for hypoglycaemic crises as CKD worsens, most randomized trials suggest that intensive glycaemic control is needed to significantly slow down the decline in function renal and albuminuria.15 The largest meta-analysis including data from the ADVANCE, ACCORD, UKPDS, and VADT trials confirmed that intensive glycaemic control was associated with a −20% risk of composite renal endpoints (worsening of filtration, UACR, and need for dialysis) essentially driven by a reduction in the risk of albuminuria. This reduction was proportional to the degree of UACR and significant for each value investigated and present at baseline.16 In therapeutic management, dose adjustments are therefore necessary as the disease progresses. But more recently, the availability of innovative drugs such as gliflozines and incretins offers more reassuring therapeutic options intended to modify the course of CKD in patients with diabetes. The 2022 American Diabetes Association guidelines give precise indications for the use of type 2 sodium glucose co-transporter inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonist (GLP-1ra) in patients with diabetes with CKD and/or cardiovascular disease.16 Both classes are equivalent, diversifying the choice based on the comorbidities present along the course of the diabetic disease, favouring SGLT2i in case of heart failure and MRC with proteinuria, GLP-1ra in case of heart attack or stroke.17
The new: the gliflozines
Recently, ‘SGLT2i’ commonly called ‘gliflozines’ have strongly imposed themselves for their ability to slow down the progression of CKD, particularly in patients with type 2 diabetes and albuminuria. Indeed, the recent ADA and EASD guidelines recommend SGLT2 as drugs of first choice in all patients with type 2 diabetes and risk of CKD progression regardless of the presence of cardiovascular disease.
The meta-analysis by Neuen et al.18 (38 723 patients enrolled from the CREDENCE, CANVAS, EMPA-REG OUTCOME, and DECLERE-TIMI trials) demonstrated that SGLT2i use carries a −33% composite renal risk (need for dialysis or transplant and CKD-related death) compared with placebo. Of great importance is the fact that the benefits of SGLT2i were statistically significant regardless of the extent of filtration at enrolment.
Finally, extraordinarily significant data are offered to us, specifically, by the DAPA-CKD trials and the EMPA-KIDNEY.
The DAPA-CKD study (∼4300 patients enrolled in optimized therapy, randomized to dapagliflozin 10 mg vs. placebo) demonstrated that in addition to optimal therapy, dapagliflozin reduced the risk of all-cause mortality by 31% overtime, and the composite relative risk of worsening renal function, end-stage renal disease, or CKD-related death was 39% of benefit of dapagliflozin vs. placebo on the composite renal outcome, regardless of the presence or absence of diabetes. The drug was well tolerated and easy to manage, and there was no significant difference in adverse events between the two study arms.19
More recently, the EMPA-KIDNEY study, the largest nephroprotection study ever conducted to date with over 6600 patients enrolled, also confirmed extraordinary efficacy and tolerability for empagliflozin, showing a −28% reduction in CKD progression and related cardiovascular mortality. The study was terminated earlier than expected due to evidence of demonstrated benefits on renal endpoints and CKD-related mortality even though it did not reach significance on the reduction in all-cause mortality.20
The relevance of these data marks an epochal turning point in the treatment of CKD, a pathology which, it is useful to remark, involves a very high mortality which, at 5 years for the patient on dialysis, is 50% higher when compared with that of, for example, lymphomas or prostate cancer.
The incretins
Glucagon-like peptide 1 receptor agonists, commonly referred to as incretins, are the other class of antidiabetic drugs that have recently been shown to be able to slow the decline in CKD and improve its renal outcomes. In the meta-analysis by Kristensen et al.21 comprising five trials (ELIXA, LEADER, SUSTAIN-6, EXSCEL, and REWIND), GLP-1ra showed a −17% reduction in the renal composite endpoint (new-onset UACR > 300 mg/g, doubling of serum creatinine, need for dialysis, and CKD-related death) with a hazard ratio (HR) of 0.83 (95% confidence interval (CI), 0.78–0.89).
However, it was noted that when only the more restrictive and specific worsening of renal function data, such as doubling of serum creatinine and need for dialysis, were taken into account, the significance was not maintained (HR 0.87, 95% CI, 0.73–1.03). Although there are currently no trials directly comparing SGLT2i and GLP-1ra on renal outcomes, the Zelniker meta-analysis (including eight trials on renal and cardiovascular endpoints) showed a −38% (HR 0.62, 95% CI, 0.58–0.67) reduction in the risk of worsening of renal function of SGLT2i vs. −18% (HR 0.82, 95% CI, 0.75–0.89) evidenced by GLP-1ra.22 In the light of these data, SGLT2i appear more effective than GLP-1ra in reducing the progression of CKD and should be preferred in the suggested therapeutic algorithm (Figure 2).

Finally, the data that are beginning to appear on the benefits of the combined use of these two classes of drugs in patients with diabetes with atherosclerotic disease and heart failure are very promising. When GLP-1ra was added to SGLT2i, a reduction in the risk of atherosclerotic events (all-cause mortality, heart attack, and stroke) was demonstrated, but no effect on the risk of heart failure.23
Funding
No funding was obtained for this study.
Data availability
The data underlying this article are available in the article.
References
Author notes
Conflict of interest: none declared.
- myocardial infarction
- hemodialysis
- diabetes mellitus
- renin-angiotensin-aldosterone system
- renal function
- cerebrovascular accident
- ischemic stroke
- kidney failure, chronic
- heart failure
- diabetes mellitus, type 2
- kidney failure
- cardiovascular system
- dialysis procedure
- kidney
- mortality
- incretin
- albuminuria



