-
PDF
- Split View
-
Views
-
Cite
Cite
Gabriele Zanotto, Alessandro Capucci, HeartInsight: from SELENE HF to implementation in clinical practice, European Heart Journal Supplements, Volume 25, Issue Supplement_C, May 2023, Pages C337–C343, https://doi.org/10.1093/eurheartjsupp/suad030
Close - Share Icon Share
Abstract
There is strong evidence that remote monitoring in cardiac implantable electronic devices can detect device malfunctions earlier than conventional monitoring and that it can be useful for detecting cardiac arrhythmias, while little data are available for an improved management of heart failure (HF). HeartInsight is a new remote monitoring algorithm developed and validated in the SELENE HF study that combines information from a diverse set of sensors integrated into one alert to detect worsening HF with promising accuracy. However, the shift from detecting technical issues or arrhythmia episodes to early predicting clinical events underscores the need to understand how to properly integrate these tools into the clinical workflow by defining an organizational model and shared guidelines for the management of HF alerts. Here, we critically discuss issues that will be raised by the implementation of this ‘enhanced’ remote monitoring approach to HF care in daily clinical practice.
Introduction
Remote monitoring (RM) in cardiac implantable electronic devices (CIEDs) has been intensively investigated over the past decade, demonstrating significant benefits in terms of lead function surveillance and battery conservation, reduced incidence of inappropriate shocks, and early detection and treatment of cardiac arrhythmias.1 Based on a recent analysis of the TRUST study,2 alert-based evaluations during continuous RM with minimized appointment-based (in-person or remote) evaluations may be considered a transformative model of dynamic care, directing attention to CIED patients as and when indicated (i.e. exception-based care). This approach may lead to fewer in-person-evaluations but with enriched actionability and better achieves follow-up goals.2 The IN-TIME trial was the first to demonstrate that RM can also improve the prognosis of heart failure (HF) patients with a CIED when using a fully automated system with daily transmissions together with a structured workflow model in clinics.3 Most of the clinical alerts in IN-TIME patients were related to the early detection of atrial or ventricular tachyarrhythmia, suboptimal biventricular pacing, or device malfunction, while there was no uniform, predefined approach to the available single HF diagnostics (e.g. nocturnal and mean heart rate, heart rate variability, physical activity, and thoracic impedance) to potentially detect early signs of decompensation.3
More recently, algorithms that combine information from a diverse set of sensors integrated into one alert to detect worsening HF have been developed with improved predictive accuracy.4,5 Optimal processing of these new diagnostic tools is expected to maximize the benefit of RM, with a potential impact on the reduction of hospital admissions for HF, which remains the leading cause of death in patients with severe systolic dysfunction6 and imposes a staggering economic burden on the healthcare system.7 However, the shift from detecting technical issues or arrhythmia episodes to early prediction of clinical events underscores the need to understand how to properly integrate these tools into the clinical workflow by defining an organizational model and shared guidelines for the management of HF alerts. Here we critically discuss issues that will be raised by the implementation of this ‘enhanced’ RM approach to HF care in daily clinical practice.
HeartInsight
HeartInsight (Biotronik SE & Co. KG, Berlin, Germany) is an algorithm that processes RM data daily transmitted from the implanted device to a Home Monitoring Service Centre (HMSC) to provide an early alert of impending episodes of worsening HF. HeartInsight can be enabled in patients with implantable cardioverter-defibrillators (ICDs) with atrial sensing capability (dual-chamber ICD or DX ICD) and in patients with cardiac resynchronization therapy defibrillators (CRT-Ds), in the absence of long-lasting/permanent atrial fibrillation. The algorithm score is based on numerical processing and a combination of baseline clinical parameters and temporal trends of seven monitored variables: 24-hour and nocturnal mean heart rate, atrial fibrillation burden, daily level of physical activity, heart rate variability, ventricular extrasystoles, and thoracic impedance.
The algorithm alerts clinical staff to an increased risk of worsening patient condition by a standard home monitoring (HM) notification and provides all relevant HF diagnostics in a dashboard integrated into the HM platform, including the long-term trend of the score (Figure 1). An automatic alert is triggered when three consecutive remote transmissions indicate a score that is equal to or higher than the programmed nominal threshold (NT). The alert is reset when the HeartInsight score spontaneously drops below a recovery threshold (calculated as NT—10). An NT of 45 is recommended (default setting). Potential adjustments can be considered under the following conditions:
NT may be decreased to increase sensitivity and alert time in patients with multiple comorbidities when the consequences of acute decompensation may be severe.
NT may be increased to reduce the rate of false positive alerts when the average trend values of HeartInsight are persistently close to the default NT, triggering frequent alerts without clinical significance.
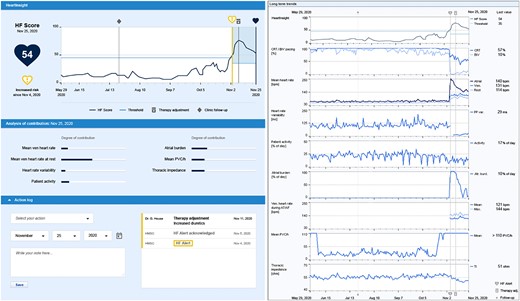
The HeartInsight dashboard on the home monitoring platform provides the current HF score, alert date, the trend of HF score with HeartInsight threshold and the current risk status, an analysis of parameters contributing to the current score, an action log to keep track of the actions taken by the clinic in response to the alert, and trends of all available heart failure diagnostics, including the percentage of cardiac resynchronization therapy delivery, mean heart rates, heart rate variability, patient activity, atrial arrhythmia burden, ventricular heart rate during atrial arrhythmia, the number of premature ventricular contractions per hour, and thoracic impedance. AT, atrial tachycardia; AF, atrial fibrillation; BiV, biventricular; bpm, beats/minute; CRT, cardiac resynchronization therapy; HF, heart failure; PVC, premature ventricular contractions.
SELENE HF study
HeartInsight was developed and validated in the SELENE HF (Selection of potential predictors of worsening HF) study.5 This observational, multi-centre, event-driven study was designed to prospectively collect follow-up and RM data trends in ICD and CRT-D patients, to document HF hospitalizations, and correlate them with RM data.8 The study cohort comprised 918 patients with a median follow-up of 22.5 months, who were randomly assigned to a derivation and a validation group prior to data analysis.
The prediction algorithm was developed using adjudicated HF hospitalizations (the primary endpoints) in the derivation cohort. Seven longitudinal parameters (24-hour and resting mean heart rate, heart rate variability, patient physical activity, burden of atrial high-rate episodes, number of ventricular extrasystoles per day, and thoracic impedance) were combined with a baseline stratifier (the Seattle HF Model9) based on clinical variables. All longitudinal parameters are known to be related to patient HF status at varying degrees of correlation. The originality of the analysis lies in the way the temporal trends of these variables have been numerically processed and combined into one numerical index whose predictive power can be precisely estimated and tested. Furthermore, the use of the Seattle HF Model (SHFM) resulted in a better adjustment of the algorithm to the patient’s individual risk profile. In the validation group, the predictor was estimated to provide correct alerts in 66 of the first post-implant HF hospitalizations within a median prediction time of 42 days, with an unexplained alerts rate below 1 per patient-year.
Organizational models
Since the introduction of RM technologies, it has become clear that their efficient use requires changes in the organizational model of CIED clinical follow-up and the definition of an RM team with specified roles and responsibilities.1 Despite this awareness, there is still significant variability in the use of RM in different hospitals, and the implementation of standardized organizational proposals is challenged by resource allocation.10,11 The availability of new diagnostic tools for HF care, such as HeartInsight, encourages sites to reassess their organizational model adopted during patient follow-up. Two main models for routine RM have been described in the literature: ‘primary nursing’ and ‘supervised network’.
The primary nursing model demonstrated its effectiveness in the HomeGuide registry12 and has been accepted as a national standard by the Italian Association of Arrhythmology and Cardiac Pacing.11 In this model, individual patients are assigned to specific groups supervised by a unique user (possibly with backup). In a clinic/hospital, multiple users can supervise many groups of patients. Normally, in a primary nursing model, users are allied health professionals (nurses or technicians) with adequate skills in device follow-up and data interpretation. They are responsible for reviewing RM transmissions, providing routine, dietary, and lifestyle recommendations, and minor medical decisions in some countries. Medical decisions generally include patient selection, unscheduled in-person visit, adjustment of medical therapy, prescription of diagnostic examinations, surgical interventions, and hospital admission. Users regularly check the HMSC and alert states, take care of and acknowledge new alerts, ask for electrophysiological/medical consultation, and react according to a pre-specified protocol.
In a supervised network, groups of remotely monitored patients can be assigned to external clinics or practitioners (spokes) from a central HMSC (hub). This model is often used when the implanting centre (hub centre) is not the same centre where the patient is followed (spoke). External groups should be managed similarly to primary nursing, with the addition of a supervisor from the hub centre. The supervisor continuously monitors that external users acknowledge and react to alerts. Supervisors also provide expert advice for troubleshooting. This model was first tested in the MoniC study,13 with promising results, and is considered a key success factor for the randomized IN-TIME trial.3
The two models are not mutually exclusive and can coexist within a single hub centre and across a network of centres. A general scheme is shown in Figure 2. The scheme may be adapted according to local requirements or needs. These models should be defined and implemented before using HeartInsight.
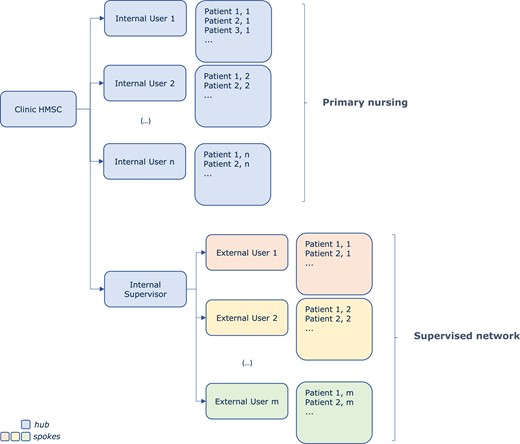
Schematic representation of primary nursing and supervised network models. HMSC, Home Monitoring Service Centre.
Patient education and enrolment
The use of HeartInsight should not modify the preliminary tasks related to the RM enrolment of the patient. The concept of remote follow-up should be presented to the patient before CIED implantation as part of the patient education and informed consent process. If HeartInsight is activated, it is important to introduce this feature to the patient, informing them that they could be contacted during follow-up to obtain information on their clinical status and not only for technical issues related to the device. Furthermore, HeartInsight-specific tasks include patient screening, algorithm activation, assessment of alert arming at the end of the run-in period (30 days after activation), and reaction to alerts.
HeartInsight has been validated in patients with standard indication for ICD or CRT-D implantation, ventricular dysfunction (ejection fraction ≤35%), and relatively stable HF condition (functional NYHA Class II or III) at the time of implantation. The algorithm is not intended to be used in patients with devices without atrial sensing capability, long-standing or permanent atrial fibrillation, or insufficient coverage of mobile phone service at home.
HeartInsight activation
Ideally, HeartInsight should be activated immediately after device implantation at hospital discharge, although it may be activated at any time during RM. The activation is not performed by the user with the support of the device programmer, but directly on the HMSC platform by the manufacturer’s service. An important user responsibility is to ensure that the thoracic impedance has been programmed to be on, as this is a mandatory requirement for HeartInsight calculation. For de novo implants, HeartInsight needs a 30-day run-in period after activation to collect the minimum required data. To improve the prediction accuracy by reducing false positive alerts, the user is encouraged to enter the clinical data required for the SHFM into the HMSC. SHFM requires about 20 clinical parameters, including demographics, therapy, and laboratory values.9 The system tolerates some missing clinical data.
Managing HeartInsight alerts: in-clinic workflow and open questions
Acute HF refers to the rapid or gradual onset of symptoms and/or signs of HF, leading to an unplanned hospital admission or emergency department visit with initiation or intensification of therapies. Diagnostic workup and subsequent management of acute decompensation episodes should start from signs or symptoms suspected of HF.14 HeartInsight can anticipate the HF crisis a median of 42 days in advance, when the patient’s symptoms may not yet be manifest. Such a relatively long interval from alerting to expected hospitalization allows sufficient time for assessments and appropriate reactions but raises a new scientific debate about the management of initial worsening in a setting of chronic HF. The continuous optimization of care (health maintenance approach) which has been considered so far, could now be improved by a strategy of early prediction of acute exacerbations as the main objective of telemedicine in HF patients with implanted devices.15
Reaction to an alert depends on the specific model implemented in the clinic. There are three possible in-clinic workflow models, each specifying a different alert management pathway, although hybrids of these are also possible.
In a very simple model where the user is also responsible for medical decisions (model A in Figure 3), the user reacts directly after evaluation of the patient profile and the HM/HeartInsight trends. Consultation with other electrophysiologists and medical or HF practitioners may be considered.
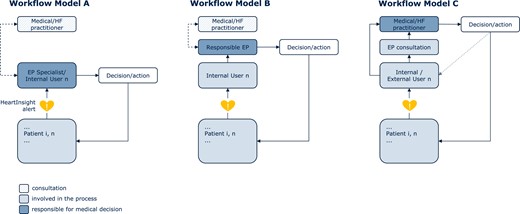
In-clinic workflow models for managing HeartInsight alerts. EP, electrophysiologist; HF, heart failure.
In a second primary nursing model (model B in Figure 3), the user is not responsible for medical decisions and consultation with a responsible electrophysiologist is mandatory. The responsible electrophysiologist may consult with a medical/HF practitioner in his/her group. The user will react according to specific instructions received directly from the responsible electrophysiologist or according to a pre-specified action plan.
Finally, model C (Figure 3) can be implemented either in primary nursing or a supervised network. With this workflow, the (internal) user is not responsible for medical decisions. After the initial evaluation of an alert and consultation with an electrophysiologist, the notification is forwarded to an internal or external medical/HF practitioner, who is responsible for further assessments, medical decisions, and follow-up. Communications may be facilitated by functionalities of the system such as the ability to add further users, share pdf file reports, or automatic electronic health record integration. The external medical/HF practitioner should inform the internal user about the relevant decisions and actions taken.
The management of a HeartInsight alert should be based on an ‘alert–assess–action’ concept and includes steps as summarized in Table 1. Ideally, within one working day from the alert, a complete remote follow-up should be performed to review the full information provided by HMSC. First, device-related technical issues should be excluded, as they may affect the algorithm (e.g. atrial oversensing, capture failures, low percentage of CRT delivery, persistent atrial pacing, etc.). Second, it is recommended to check HM compliance as frequent transmission gaps may reduce available information processed by the algorithm (alerts are eventually disabled with <55% compliance in 90 days). The changes in cardiac arrhythmia status should then be verified and, eventually, a careful clinical risk assessment of the alert should be performed. To evaluate the patient’s status, the HeartInsight dashboard is the primary source of information, providing the current value and long-term trend of the score, the analysis of parameters that contribute to the score, the long-term trends of all variables related to HF, and the action log for information on previous actions taken (Figure 1). After the initial assessment, the system allows also to postpone an alert up to 21 days for subsequent evaluation.
Response to a HeartInsight alert and alert–assess–action as recommended steps
| Alert check . | Assess through patient contact . | Action upon evaluation of information . | |
|---|---|---|---|
| Access the HMSC for a complete remote follow-up and to review full information provided by the HeartInsight and other parameters Ideally, the assessment should be done within 1 working day from the alert | |||
| Exclude device-related technical issues | Device-related technical issues or inadequate device programming may affect HeartInsight alerts (e.g. atrial oversensing causing inappropriate detection of AF or PVC, capture failures, low CRT%, persistent atrial pacing, etc.) | Contact the patient | In-clinic visit if necessary |
| Exclude deterioration of HM compliance | Low HM compliance (frequent gaps in transmissions) may reduce the information processed by HeartInsight. Alerts are automatically disabled with <55% compliance in 90 days | Contact the patient in 1–2 working days |
|
| Check changes in AF status (first episode detection, significant burden increase, etc.) | Therapy adjustment may be urgently needed for rhythm control and thromboembolic risk mitigation If AF turns to permanent, HeartInsight needs to be disabled |
| Evaluate therapy adjustment, further examinations |
| Check clinical risk of alert and evaluate HeartInsight and parameter temporal trends | Evaluation of patient profile, collection of information related to the patient’s condition, analysis of the degree of contribution of individual components to the HeartInsight alert | Contact the patient in 1–2 working days to Conduct patient interview | Evaluate appropriate medical actions at this stage |
| Alert check . | Assess through patient contact . | Action upon evaluation of information . | |
|---|---|---|---|
| Access the HMSC for a complete remote follow-up and to review full information provided by the HeartInsight and other parameters Ideally, the assessment should be done within 1 working day from the alert | |||
| Exclude device-related technical issues | Device-related technical issues or inadequate device programming may affect HeartInsight alerts (e.g. atrial oversensing causing inappropriate detection of AF or PVC, capture failures, low CRT%, persistent atrial pacing, etc.) | Contact the patient | In-clinic visit if necessary |
| Exclude deterioration of HM compliance | Low HM compliance (frequent gaps in transmissions) may reduce the information processed by HeartInsight. Alerts are automatically disabled with <55% compliance in 90 days | Contact the patient in 1–2 working days |
|
| Check changes in AF status (first episode detection, significant burden increase, etc.) | Therapy adjustment may be urgently needed for rhythm control and thromboembolic risk mitigation If AF turns to permanent, HeartInsight needs to be disabled |
| Evaluate therapy adjustment, further examinations |
| Check clinical risk of alert and evaluate HeartInsight and parameter temporal trends | Evaluation of patient profile, collection of information related to the patient’s condition, analysis of the degree of contribution of individual components to the HeartInsight alert | Contact the patient in 1–2 working days to Conduct patient interview | Evaluate appropriate medical actions at this stage |
Upon finalizing this scheme one can then either acknowledge the alert or postpone in order to be re-alerted for further assessment of the trajectory of the patient.
AF, atrial fibrillation; CRT, cardiac resynchronization therapy; HM, Home Monitoring; HMSC, Home Monitoring Service Centre; PVC, premature ventricular contractions.
Response to a HeartInsight alert and alert–assess–action as recommended steps
| Alert check . | Assess through patient contact . | Action upon evaluation of information . | |
|---|---|---|---|
| Access the HMSC for a complete remote follow-up and to review full information provided by the HeartInsight and other parameters Ideally, the assessment should be done within 1 working day from the alert | |||
| Exclude device-related technical issues | Device-related technical issues or inadequate device programming may affect HeartInsight alerts (e.g. atrial oversensing causing inappropriate detection of AF or PVC, capture failures, low CRT%, persistent atrial pacing, etc.) | Contact the patient | In-clinic visit if necessary |
| Exclude deterioration of HM compliance | Low HM compliance (frequent gaps in transmissions) may reduce the information processed by HeartInsight. Alerts are automatically disabled with <55% compliance in 90 days | Contact the patient in 1–2 working days |
|
| Check changes in AF status (first episode detection, significant burden increase, etc.) | Therapy adjustment may be urgently needed for rhythm control and thromboembolic risk mitigation If AF turns to permanent, HeartInsight needs to be disabled |
| Evaluate therapy adjustment, further examinations |
| Check clinical risk of alert and evaluate HeartInsight and parameter temporal trends | Evaluation of patient profile, collection of information related to the patient’s condition, analysis of the degree of contribution of individual components to the HeartInsight alert | Contact the patient in 1–2 working days to Conduct patient interview | Evaluate appropriate medical actions at this stage |
| Alert check . | Assess through patient contact . | Action upon evaluation of information . | |
|---|---|---|---|
| Access the HMSC for a complete remote follow-up and to review full information provided by the HeartInsight and other parameters Ideally, the assessment should be done within 1 working day from the alert | |||
| Exclude device-related technical issues | Device-related technical issues or inadequate device programming may affect HeartInsight alerts (e.g. atrial oversensing causing inappropriate detection of AF or PVC, capture failures, low CRT%, persistent atrial pacing, etc.) | Contact the patient | In-clinic visit if necessary |
| Exclude deterioration of HM compliance | Low HM compliance (frequent gaps in transmissions) may reduce the information processed by HeartInsight. Alerts are automatically disabled with <55% compliance in 90 days | Contact the patient in 1–2 working days |
|
| Check changes in AF status (first episode detection, significant burden increase, etc.) | Therapy adjustment may be urgently needed for rhythm control and thromboembolic risk mitigation If AF turns to permanent, HeartInsight needs to be disabled |
| Evaluate therapy adjustment, further examinations |
| Check clinical risk of alert and evaluate HeartInsight and parameter temporal trends | Evaluation of patient profile, collection of information related to the patient’s condition, analysis of the degree of contribution of individual components to the HeartInsight alert | Contact the patient in 1–2 working days to Conduct patient interview | Evaluate appropriate medical actions at this stage |
Upon finalizing this scheme one can then either acknowledge the alert or postpone in order to be re-alerted for further assessment of the trajectory of the patient.
AF, atrial fibrillation; CRT, cardiac resynchronization therapy; HM, Home Monitoring; HMSC, Home Monitoring Service Centre; PVC, premature ventricular contractions.
The patient interview is also an important part of the assessment process. The interview should be conducted by an expert user (including the nurse, referring electrophysiologist, or medical practitioner) in a reassuring way to complete specific assessments. Based on a previously reported scheme,16 the interview should address the following objectives:
Assess ongoing therapy: compliance to the prescribed therapy, appropriateness of therapy, and dosage.
Assess recent conditions (fever, medical visits, hospitalizations for any reason, etc.) or significant lifestyle changes, with a special focus on diet and activity.
Assess variations in body weight and other symptoms potentially indicative of acute worsening HF: swelling in the legs, worsening breathlessness, fatigue, and sleep quality.
It is important to emphasize that symptoms should be reviewed but may not be manifest at the time of HeartInsight alert. Although there is no data to support any specific recommendation in the absence of signs of decompensation, medical actions should certainly be based on the individual patient profile, and we provide a reference list of basic elements in the Supplementary material online, to facilitate interpretation and medical decisions. After the patient interview and based on the collected information, some possible reactions to consider are (i) improving medication compliance, (ii) advising lifestyle changes, (iii) modifying the medication, (iv) hospitalization or scheduling a clinical visit, and (v) re-evaluation of the patient in case of no actionable findings. After an initial patient contact or if therapy has been changed, regular reviews of the HeartInsight component trends and other HM variables should continue as deemed necessary (new contact, therapy adjustments, in-clinic visits, diagnostic examinations, etc.) or until the patient exits the high-risk status (recovery). This can easily be achieved by using the ‘postpone’ functionality to receive automatic ‘re-alerts’ after a programmable number of days or weeks unless the alert is reset. Re-evaluation and update of the SHFM score may also be useful after the alert reset.
Conclusions
HeartInsight is a new HF management solution with promising prediction accuracy characterized by a relatively long interval from alert to expected severe symptoms. We recommend identifying an appropriate organizational model with pre-specified roles and responsibilities and an in-clinic workflow to manage HF alerts to be implemented in routine daily practice before using this algorithm. Prospective clinical studies (currently under design) are needed to assess the incremental value of this ‘enhanced’ RM strategy for HF care, before testing the whole system (algorithm plus workflow) in randomized clinical trials.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
The authors thank Dejan Danilovic for critical reading and editing the manuscript.
Funding
None declared.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Author notes
Conflict of interest: None declared.



