-
PDF
- Split View
-
Views
-
Cite
Cite
Fiorenzo Gaita, Natascia Cerrato, Andrea Saglietto, Domenico Caponi, Leonardo Calò, Carla Giustetto, The Brugada syndrome: risk stratification, European Heart Journal Supplements, Volume 25, Issue Supplement_C, May 2023, Pages C27–C31, https://doi.org/10.1093/eurheartjsupp/suad035
Close - Share Icon Share
Abstract
Thirty years after its first description, the knowledge regarding Brugada syndrome has greatly increased. Spontaneous type 1 ECG pattern (BrECG) is a well-defined prognostic marker in asymptomatic patients and is associated with a double risk of arrhythmic events during follow-up as compared to drug-induced ECG pattern. Due to the extreme variability of the ECG pattern over time, the spontaneous type 1 BrECG must be carefully sought, not only through periodic ECGs but especially with repeated 12-lead 24-h Holter monitoring, with V1 and V2 electrodes placed also on the second and third intercostal space, in order to explore the right ventricular outflow tract. 12-lead 24-h Holter should also be performed in all the patients with a dubious BrECG pattern even before the drug challenge with sodium channel blockers, which carries a low but definite risk of complications. In addition to spontaneous type 1, other electrocardiographic markers of increased arrhythmic risk have been described, such as first-degree AV block, QRS fragmentation, S wave in lead I and II, and increased QRS duration. The electrophysiological study in asymptomatic patients with a spontaneous ECG Brugada pattern is still under jury and further studies need to clarify its precise role.
Introduction
Thirty years after the description of the first eight cases of ‘right bundle branch block, persistent ST-segment elevation, and sudden cardiac death’,1 the knowledge regarding the Brugada syndrome has greatly increased. The diagnosis is based on the documentation of the typical ECG pattern, characterized by a J-point elevation ≥ 2 mm, coved-type ST-segment elevation, and negative T wave in one or more right precordial leads, called type 1 Brugada ECG (BrECG) (Figure 1(A)). One possible mechanism which can explain the ECG manifestations is the imbalance between the inward sodium (Na+) and calcium (Ca++) currents and the transient outward potassium (K+) current (Ito). This occurs predominantly in the right ventricular epicardium: in fact, Ito is much more represented in the right ventricle and, particularly, in the right ventricular outflow tract (RVOT), than it is in the left ventricle.2
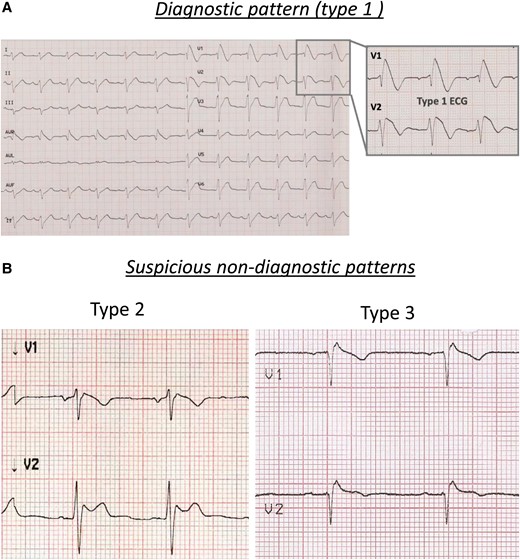
Brugada electrocardiographic patterns: (A) diagnostic pattern (type 1) and (B) suspicious pattern (type 2, left panel; type 3, right panel).
Differential diagnosis must be considered from a wide variety of conditions that present ST-segment elevation in the right precordial leads, such as pericarditis, acute myocardial ischaemia, mechanical compression of the RVOT (such as pectus excavatum), hyperkaliemia, hypercalcemia, hypothermia, pulmonary embolism, early repolarization, and right bundle branch block.
The position of the RVOT in the thorax varies from one individual to another. Therefore, it is important to explore the RVOT by placing the electrodes not only in the standard fourth intercostal space but also in a higher position (second and third intercostal space) to increase the sensitivity of the ECG to detect the diagnostic Brugada type 1 ECG pattern.2–4 Moreover, it is important to note that several factors can affect the BrECG pattern, such as heart rate, vagal nerve stimulation, post-prandial period, glycemic and insulin levels, fever, exercise, and drug intake. Therefore, the ECG pattern is variable over time, leading to the presence of diagnostic ECGs alternated with suspicious or normal ECGs in the same patient.
In the past, what we now define as suspicious patterns were described as ‘type 2’ (≥2 mm J-point elevation, ≥ 1 mm ST-segment elevation with saddleback appearance, followed by a positive or biphasic T-wave) or ‘type 3’ (either a saddleback or coved-type ST-segment elevation < 1 mm) (Figure 1(B)). In the presence of such dubious patterns, the most common method to unmask the diagnostic ECG is the drug challenge with sodium channel blockers (intravenous ajmaline 1 mg/kg in 5–10 min or flecainide 2 mg/kg over 10 min), which has a sensitivity around 80%.5 Beyond its diagnostic value, the drug test can provide additional information. In fact, a shorter time to positivity has recently been reported as a clinical parameter that predicts subsequent spontaneous type 1 documentation during follow-up6 and the interval between the onset of the drug-induced coved-type ST-segment elevation and its termination at the level of the isoelectric line, in leads V1 and V2, has been reported to predict ventricular arrhythmias inducibility at the electrophysiological study (EPS).7 However, the test is burdened by rare, but potentially serious complications, such as the development of life-threatening ventricular arrhythmias in about 1.8% of patients, sometimes refractory to external defibrillation and, in extreme situations, requiring extracorporeal circulation.8
Spontaneous type 1 Brugada pattern
Although we consider as Brugada patients both those with spontaneous and those with drug-induced ECG pattern, these two groups of patients are completely different from a prognostic and therapeutic point of view. All the authors agree that in addition to the history of aborted SCD [hazard ratio (HR) 10] or unexplained syncope (HR 3.7), a spontaneous type 1 BrECG pattern is a risk factor for future arrhythmic events (HR 2.7) as compared to the drug-induced ECG pattern.9 In the FINGER study,10 in which our group participated, patients with spontaneous type 1 BrECG pattern had a risk of SCD of 2.3% vs. 1% in patients with drug-induced type 1 BrECG pattern, at a mean follow-up of 32 months. This doubled risk of arrhythmic events is still present when stratifying the population according to symptoms at clinical presentation: in fact, in patients with a previous history of aborted SCD or syncope, the annual risk was respectively 10% per year and 2.3% per year in patients with spontaneous BrECG, while in patients with drug-induced ECG pattern it was halved to 5.2% per year and 1.5% per year, respectively.
In symptomatic patients, the distinction between spontaneous and drug-induced pattern is not so relevant for the therapeutic management, because there is general agreement that all these patients must be treated. On the other side, this distinction is crucial in the asymptomatic subjects, as there is still no clear consensus on how to stratify and manage this population. Despite the risk of arrhythmic events is low in asymptomatic patients, it is still twice as high in patients with spontaneous type 1 compared to those with drug-induced type 1 (0.8% per year vs. 0.35% per year).10 For this reason, it is important to systematically and accurately search for spontaneous type 1 pattern in this population.
At the beginning, repeated ECG recordings at different times have been suggested to increase the probability of detecting a spontaneous type 1 ECG. A previous work11 showed that, as expected, the likelihood of documenting the spontaneous type 1 BrECG pattern was higher when the number of electrocardiographic recordings during a year was increased. In the last 10 years, the advent of the 12-lead 24-h Holter monitoring has significantly increased the possibility of identifying the presence of a spontaneous type 1. In a previous report,12 our group demonstrated that in patients with only drug-induced BrECG pattern, the 12-lead Holter monitoring allowed to identify at least 20% of subjects with also a spontaneous type 1 BrECG (Figure 2), who would have been considered only induced and therefore at low risk based on periodic 12-lead ECGs. Increasing the recording time by repeating 12-lead Holter monitoring, it is possible to identify the type 1 pattern in a further 15% of subjects, who were negative at the first 12-lead Holter monitoring. These results confirmed the importance of the duration of electrocardiographic recording to increase the sensitivity in detecting spontaneous type 1 BrECG. Interestingly, this study also showed that there were circadian fluctuations of the BrECG pattern, with the majority of patients presenting spontaneous type 1 BrECG between 12-noon and 12-midnight.12
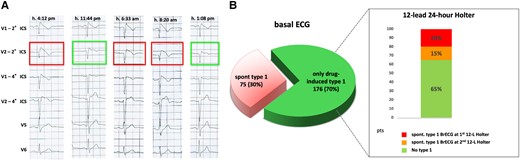
12-lead 24-h Holter monitoring in patients with Brugada electrocardiographic pattern: (A) intra-daily fluctuations of the electrocardiographic pattern, oscillating between diagnostic type 1 (1st, 3rd and 4th strip) and suspicious non-diagnostic patterns (2nd and 5th strip); (B) role of 12-lead Holter monitoring in unmasking spontaneous diagnostic type 1 pattern in patients originally presenting with drug-induced only type 1 pattern (adapted from Cerrato et al.12).
Electrocardiographic markers of increased arrhythmic risk
In addition to the spontaneous type 1 pattern, other ECG parameters should be sought, especially in asymptomatic patients, as they are associated with an increased arrhythmic risk:13,14
In the work by Calò et al.,21 S wave ≥40 ms in lead I was present in nearly all patients with a history of SCD or aborted SCD (97%) but, despite a good sensitivity, this parameter lacked specificity given that it was also present in the 56% of the remaining population. In a subsequent work,22 a QRS duration ≥110 ms in lead II and/or V6 and/or S-wave duration ≥40 ms in lead I and/or II were showed to be significant risk factors for the occurrence and timing of the first arrhythmic event and its recurrences. All these ECG parameters are an expression of a conduction delay at the level of the RVOT. This is confirmed by the fact that symptomatic patients with BrECG pattern show a higher prevalence of late potentials and is even more corroborated by the findings of the invasive epicardial mapping of RVOT,23,24 where abnormal signals, characterized by fragmented and long duration potentials, are recorded predominantly in this area. The extension of the area of abnormal electrical activity correlates with the presence of spontaneous or induced BrECG pattern and ajmaline administration increases the area of abnormal potentials, which is the target of catheter ablation.24,25
The role of electrophysiological study
The role of EPS in the risk stratification of Brugada patients is still debated. Brugada et al.24 demonstrated that induction of ventricular tachycardia/fibrillation at EPS identified a population with a higher arrhythmic risk during follow-up, but this finding was not confirmed by other studies.26–28 These conflicting results may be explained by the different protocols used and whether the EPS was performed in the presence of type 1 ECG pattern or not.
The metanalysis by Sroubek et al.29 showed that EPS with single and double extrastimulus was useful to identify patients at risk of cardiac events in the overall Brugada population. In our experience, EPS resulted particularly useful to stratify the arrhythmic risk in patients with unexplained syncope.30 In fact, patients with unexplained syncope and positive EPS showed a significantly higher risk of arrhythmic events (27%) as compared to those with negative EPS, in which no ventricular events were reported during a mean follow-up of 62 months. In the asymptomatic patients, the use of EPS is still more controversial. Although it is characterized by a low specificity, i.e. a high number of false positives, many authors agree that EPS, especially with a non-aggressive protocol (up to two extrastimuli), represents to date the only tool we have, combined with careful ECG analysis, to stratify the risk of asymptomatic Brugada patients (class IIb, 2022 European Society of Cardiology guidelines31).
Conclusions
The knowledge regarding the Brugada syndrome has greatly increased since the first description. The diagnosis is based on the typical ECG pattern characterized by a coved-type ST-segment elevation ≥ 2 mm, followed by a negative T wave in one or more right precordial leads (type 1 BrECG).
The ECG pattern must be searched by exploring the RVOT, and positioning the ECG electrodes V1 and V2 also on the second and third intercostal space. The typical ECG pattern presents a variable trend over time; therefore, it must be carefully sought by repeated 12-lead 24-h Holter monitoring, with V1 and V2 electrodes placed also on the second and third intercostal space. 12-lead 24-h Holter should also be performed in all the patients with a suspicious BrECG pattern, before the drug challenge with sodium channel blockers, which is not without complications.
The presence of the spontaneous type 1 BrECG pattern is a significant prognostic marker in asymptomatic patients and is associated with a doubled risk for arrhythmic events during follow-up as compared to drug-induced ECG pattern. Moreover, an accurate ECG analysis to find additional ECG features such as first-degree AV block, QRS fragmentation, deep and wide S wave in lead I, and QRS duration ≥ 110 ms may provide further information to stratify the arrhythmic risk.
The role of the EPS remains controversial, since, despite a good sensitivity in identifying patients at greater risk of arrhythmic events, its specificity remains low.
Risk stratification of the asymptomatic patients is a crucial point, because their risk of SCD is relatively low, while the probability of complications related to the available treatments is quite high. Today, ICD is the only therapy suggested by the guidelines31 in the asymptomatic patients with spontaneous type 1 ECG, but the long-term device-related complications (e.g. lead fracture, infections, psychological distress, and so on) in this young population can outweigh the expected benefits. Other therapeutic strategies, both pharmacological (e.g. quinidine, cilostazol, or new drugs) and ablative, could solve this issue, if they prove effective and safe in the long term.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
Author notes
Conflict of interest: None declared.



