-
PDF
- Split View
-
Views
-
Cite
Cite
Leonardo De Luca, Andrea Rubboli, Maddalena Lettino, Marco Tubaro, Sergio Leonardi, Gianni Casella, Serafina Valente, Roberta Rossini, Alessandro Sciahbasi, Enrico Natale, Paolo Trambaiolo, Alessandro Navazio, Manlio Cipriani, Marco Corda, Alfredo De Nardo, Giuseppina Maura Francese, Cosimo Napoletano, Emanuele Tizzani, Federico Nardi, Loris Roncon, Pasquale Caldarola, Carmine Riccio, Domenico Gabrielli, Fabrizio Oliva, Michele Massimo Gulizia, Furio Colivicchi, ANMCO position paper on antithrombotic treatment of patients with atrial fibrillation undergoing intracoronary stenting and/or acute coronary syndromes, European Heart Journal Supplements, Volume 24, Issue Supplement_C, May 2022, Pages C254–C271, https://doi.org/10.1093/eurheartj/suac020
Close - Share Icon Share
Abstract
Patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention (PCI) with or without acute coronary syndromes (ACS) represent a subgroup with a challenging pharmacological management. Indeed, if on the one hand, antithrombotic therapy should reduce the risk related to recurrent ischaemic events and/or stent thrombosis; on the other hand, care must be taken to avoid major bleeding events. In recent years, several trials, which overall included more than 12 000 patients, have been conducted demonstrating the safety of different therapeutic combinations of oral antiplatelet and anticoagulant agents. In the present ANMCO position paper, we propose a decision-making algorithm on antithrombotic strategies based on scientific evidence and expert consensus to be adopted in the periprocedural phase, at the time of hospital discharge, and in the long-term follow-up of patients with AF undergoing PCI with/without ACS.
Epidemiology of patients with atrial fibrillation and coronary heart disease
About a third of patients with atrial fibrillation (AF) have an associated ischaemic coronary heart disease, and 5–8% of the subjects undergoing percutaneous coronary intervention (PCI) suffer from concomitant AF.1 Similarly, AF incidence during acute coronary syndrome (ACS) varies between 2% and 23%.2
In the BLITZ-3 Study, about 6% of the patients with ACS presented AF before admission, while 6% of ACS with ST-elevation (STEMI) and 9% of non-ST-elevation (NSTEMI) developed AF during hospitalization.3
In this context, patients with AF represent a complex population, aggravated by increased mortality.4 In patients with pre-existing AF at the time of the ACS, the arrhythmia correlates to an important extent and severity of the disease, whether it be cardiac or non-cardiac.4 The case is different if AF appears during an ACS. Under these circumstances, AF is secondary to ischaemia, to the increased atrial and ventricular filling pressures, diastolic dysfunction, or neuro-hormonal activation; conditions which imply a major extension of the necrosis, a major haemodynamic impairment, aggravated by the arrhythmia, and consequently with a less favourable prognosis.2,4
Thanks to the analysis of the main Italian surveys on ACS conducted by ANMCO (National Italian Association of Hospital Cardiologists) during the years 2001–14 in Italy, it is possible to have precise data on the relationship between ACS and AF. In a recent analysis, in which 16 803 ACS patients were included, 1019 cases (6.1%) presented with concomitant AF. The prevalence was higher in patients with NSTEMI (7.2%) compared to those with STEMI (4.7%) and has not changed over the years (Figure 1). As confirmation of what is known, patients with AF were older, more often women, and more easily affected by diabetes, hypertension, and renal impairment. A major number of these AF subjects had a previous history of angina, infarction, or stroke, confirming that the presence of the arrhythmia was related to a history of heart disease.
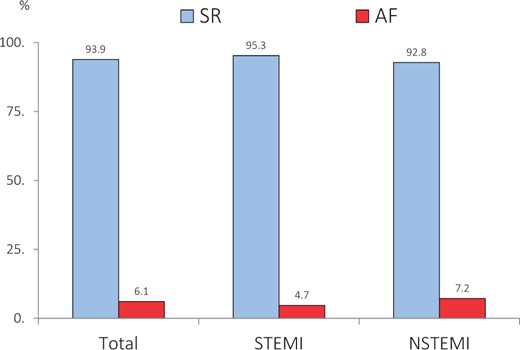
Prevalence of atrial fibrillation in the Italian ANMCO Registries (2001–14). About 16 803 patients with acute coronary syndrome hospitalized in the Italian CCUs. Modified from Ref.5
Generally, AF patients undergo a lower number of PCI and are undertreated compared with patients that do not suffer from AF2 and this poor use of effective treatments has always been considered a justification for their worst prognosis. However, the analysis documented, in the 14 years of observation, a progressive increase of the use of coronary angiography and PCI in patients with ACS and AF, independently of the type of infarction.5 In NSTEMI, the number of coronary angiography grew from 28.6% reported in the BLITZ-1 Study to 61.7% in the EYESHOT Study and from 10.2% to 40.6% of PCIs; for STEMI from 40% to 89.3% of coronary angiography and from 32.3% to 80.4% of PCI, respectively. The analysis of the Italian registries also presented evidence of a substantial reduction in-hospital mortality in all the groups during the 14-year observation period, also evident in AF patients, although less significantly (Figure 2). It is plausible that the major use of revascularization in AF patients has favourably influenced in-hospital mortality.5
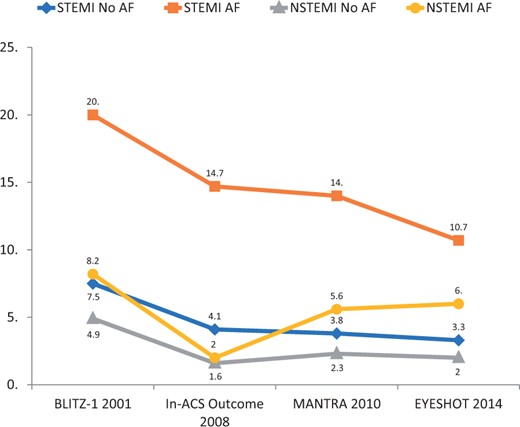
Hospital mortality observed in the Italian ANMCO Registries in subjects with acute coronary syndrome (STEMI or NSTEMI) with or without atrial fibrillation. Modified from Ref.5
However, patients with ACS and AF continue to demonstrate at least twice the high mortality compared with subjects without AF, demonstrating that the arrhythmia is present in subjects with a greater burden of pre-existing pathology or with acute haemodynamic impairment.
It is, therefore, of fundamental importance that drug therapy, and in particular antithrombotic therapy, in addition to reperfusion strategies, should be given to these patients from the early intra-hospital stages to long-term follow-up.
Intra-procedural management of antithrombotic therapy
The optimal periprocedural management of patients with AF undergoing PCI, requiring combined oral anticoagulant therapy (OAT) and antiplatelet drugs, requires a personalized approach that considers not only drug medication (type, dosages, and timing of administration and suspension) but also vascular access (Figure 3).
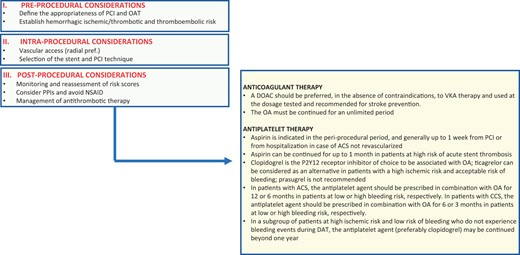
Algorithm for the management of patients with atrial fibrillation and indication for anticoagulant therapy undergoing coronary angioplasty. ACS, acute coronary syndrome; CCS, chronic coronary syndrome; DAT, double antithrombotic therapy; DOAC, direct oral anticoagulants; NSAID, non-steroidal anti-inflammatory drugs; OA, oral anticoagulant; PCI, coronary angioplasty; PPIs, proton pump inhibitors; VKA, vitamin K antagonists.
In fact, in order to minimize the haemorrhagic risk, radial vascular access rather than femoral access is strongly recommended in patients on OAT. If the radial approach is not possible, then the use of an eco-guided femoral puncture and access closure systems is strongly recommended.
Aspirin
In all studies conducted so far, aspirin has been administered to AF patients undergoing PCI in the periprocedural phase and even in a dual antithrombotic therapy regimen.6–9 Therefore, and in accordance with the guidelines, low-dose aspirin (<160 mg) should always be administered at the time of admission to patients treated with PCI or in an ACS context and maintained in the periprocedural period for a maximum of 7 days, except in special cases that will be specified later.10
P2Y12 platelet receptor inhibitors
The oral antiplatelet of choice in patients already in OAT is clopidogrel, while the stronger oral P2Y12 receptor inhibitors, ticagrelor and prasugrel, are generally not recommended in combination with aspirin. Oral antiplatelet inhibitors should be administered as a loading dose (300–600 mg for clopidogrel, 180 mg for ticagrelor, and 60 mg for prasugrel) only after coronary angiography and confirmed indication to PCI.10 Cangrelor is an intravenous adenosine-diphosphate (ADP) receptor inhibitor that can be administered to patients who have not received dual antiaggregant therapy (DAPT). It has been studied in patients treated with vitamin K inhibitors (VKA) and with an international normalized ratio (INR) <1.5 in the CHAMPION programme, but no evidence is available regarding patients receiving direct oral anticoagulants (DOACs).1,11,12 Therefore, it should be reserved only for patients in whom the administration of oral drugs is not possible and the coronary thrombotic risk is considered high. In selected cases (e.g. treatment-naive patients at very high ischaemic risk), cangrelor can also be considered as a bridge to the effect of oral inhibitors administered in the periprocedural phase.
Glycoprotein IIb/IIIa inhibitors
These strong intravenous antiplatelet agents are generally not recommended in patients on OAT,13 especially when VKA or DOAC, have not been withdrawn. If oral administration of antiplatelet agents is not feasible and the coronary thrombotic load is very high, then a bolus or short infusion (<2 h) should be considered, to minimize bleeding complications.14
Anticoagulants (oral and parenteral)
Patients in OAT who need to perform urgent or emerging PCI can continue OAT, including DOACs15,-18 without interruption during the procedure.10 It is advisable to interrupt OAT at least 1 day before the procedure in patients who have to undergo elective PCI; in the case of DOAC, the type of drug and the patient’s renal function should be taken into consideration.19 Anticoagulant therapy will be resumed upon reaching complete haemostasis; in the case of patients treated with DOAC, given the rapid onset of the action, there is no need to use heparin at an early stage, which is instead required in patients treated with VKA, with INR values up to 1.8–2.0.
Based on limited and indirect evidence, parenteral anticoagulant therapy during the procedure should be administered independently from the last administration of oral anticoagulant to all patients receiving DOAC, particularly for factor Xa antagonists, and to those receiving VKA if the INR <2.5.10,20
If the patient has a new indication for OAT, the start of this therapy is uncertain. In general, considering the increased intensity and combination of periprocedural antithrombotic drugs, in patients with a new OAT indication, it is preferable to start the therapy at least 48 h after PCI and probably within 5–7 days, particularly if there is an indication for DOAC.
Evidence on antithrombotic strategies from the post-procedural phase to hospital discharge
Following the demonstration of the superiority of DAPT over OAT in the ISAR (Intracoronary Stenting And Antithrombotic Regimen Trial) and STARS (Stent Antithrombotic Regimen Study) trials, DAPT with aspirin and a P2Y12 receptor inhibitor represents the cornerstone of antithrombotic therapy in patients undergoing PCI.21,22 In contrast, OAT has always been superior to DAPT in preventing cardioembolic risk in patients with AF.23 This cultural background has led to the use, in clinical practice, of the so-called triple antithrombotic therapy (TAT) or the combination of DAPT and an oral anticoagulant drug in the treatment of patients with AF undergoing PCI, in order to prevent ischaemic/thrombotic events related to coronary artery disease or PCI and thromboembolic events associated with AF. In 2010, for the first time, a consensus document was published by the European Society of Cardiology (ESC) recommending TAT with VKA, aspirin, and clopidogrel in AF patients undergoing PCI.24
At the same time, DOACs have been tested in randomized clinical trials, demonstrating a favourable risk profile compared with VKAs and being able to significantly reduce intracranial haemorrhage.25 However, the use of TAT, even in combination with DOACs, was burdened by a high incidence of haemorrhagic adverse events, including fatal ones.8 At the same time, the fear of bleeding complications has often led to the prescription of inadequate antithrombotic regimens because of an underdosage, leading to an increased risk of ischaemic and thromboembolic events.26
With the aim of reducing the risk of major bleeding associated with TAT, several trials have been designed which have compared, in the post-PCI phase, TAT with a dual antithrombotic therapy (DAT), consisting of a single oral antiplatelet drug plus a single oral anticoagulant6–9 (Table 1). These trials have assessed various combinations of antithrombotic drugs and different dosages, and have typically excluded aspirin in the DAT arm, which, as we have previously mentioned, should instead be administered during the periprocedural phase.
| . | Design . | Patients (n) . | Treatment groups . | AF (%) . | Maximum time in TAT in DTA group (days) . | ACS (%) . | Follow-up (months) . | Primary outcome . |
|---|---|---|---|---|---|---|---|---|
| WOEST | RCT, open-label, 1:1 | 573 |
| 69 | <1 | 28 | 12 | Haemorrhagic events of any kind (according to the TIMI and GUSTO criteria) |
| ISAR-TRIPLE | RCT open-label, 1:1 | 614 |
| 84 | / | 32 | 9 | Composite endpoint of death, myocardial infarction, stent thrombosis, stroke or major bleeding |
| PIONEER-AF | RCT open-label, 1:1:1 | 2124 |
| 100 | 3 | 52 | 12 | Clinically relevant bleeding (major bleeding according to TIMI criteria, minor bleeding or bleeding that required medical intervention) |
| RE-DUAL PCI | RCT open-label, 1:1:1 | 2725 |
| 100 | 5 | 51 | 14 | Clinically relevant bleeding (major bleeding according to ISTH definition or clinically relevant non-major bleeding events) |
| AUGUSTUS | RCT open-label, 2 × 2 | 4614 |
| 100 | 14 | 60 | 6 | Clinically relevant bleeding (major bleeding according to ISTH definition or clinically relevant non-major bleeding events) |
| ENTRUST-AF PCI | RCT open-label, 1:1 | 1506 |
| 100 | 5 | 52 | 12 | Clinically relevant major or non-major bleeding (according to ISTH criteria) |
| . | Design . | Patients (n) . | Treatment groups . | AF (%) . | Maximum time in TAT in DTA group (days) . | ACS (%) . | Follow-up (months) . | Primary outcome . |
|---|---|---|---|---|---|---|---|---|
| WOEST | RCT, open-label, 1:1 | 573 |
| 69 | <1 | 28 | 12 | Haemorrhagic events of any kind (according to the TIMI and GUSTO criteria) |
| ISAR-TRIPLE | RCT open-label, 1:1 | 614 |
| 84 | / | 32 | 9 | Composite endpoint of death, myocardial infarction, stent thrombosis, stroke or major bleeding |
| PIONEER-AF | RCT open-label, 1:1:1 | 2124 |
| 100 | 3 | 52 | 12 | Clinically relevant bleeding (major bleeding according to TIMI criteria, minor bleeding or bleeding that required medical intervention) |
| RE-DUAL PCI | RCT open-label, 1:1:1 | 2725 |
| 100 | 5 | 51 | 14 | Clinically relevant bleeding (major bleeding according to ISTH definition or clinically relevant non-major bleeding events) |
| AUGUSTUS | RCT open-label, 2 × 2 | 4614 |
| 100 | 14 | 60 | 6 | Clinically relevant bleeding (major bleeding according to ISTH definition or clinically relevant non-major bleeding events) |
| ENTRUST-AF PCI | RCT open-label, 1:1 | 1506 |
| 100 | 5 | 52 | 12 | Clinically relevant major or non-major bleeding (according to ISTH criteria) |
ACS, acute coronoary syndrome; AF, atrial fibrillation; bd, bis in die; GUSTO, Global Use of Streptokinase and t-PA for Occluded Coronary Arteries; ISTH, International Society on Thrombosis and Haemostasis; od, omni die; P2Y12i, P2Y12 platelet receptor inhibitors; RCT, randomized controlled trial; VKA, vitamin K antagonists.
| . | Design . | Patients (n) . | Treatment groups . | AF (%) . | Maximum time in TAT in DTA group (days) . | ACS (%) . | Follow-up (months) . | Primary outcome . |
|---|---|---|---|---|---|---|---|---|
| WOEST | RCT, open-label, 1:1 | 573 |
| 69 | <1 | 28 | 12 | Haemorrhagic events of any kind (according to the TIMI and GUSTO criteria) |
| ISAR-TRIPLE | RCT open-label, 1:1 | 614 |
| 84 | / | 32 | 9 | Composite endpoint of death, myocardial infarction, stent thrombosis, stroke or major bleeding |
| PIONEER-AF | RCT open-label, 1:1:1 | 2124 |
| 100 | 3 | 52 | 12 | Clinically relevant bleeding (major bleeding according to TIMI criteria, minor bleeding or bleeding that required medical intervention) |
| RE-DUAL PCI | RCT open-label, 1:1:1 | 2725 |
| 100 | 5 | 51 | 14 | Clinically relevant bleeding (major bleeding according to ISTH definition or clinically relevant non-major bleeding events) |
| AUGUSTUS | RCT open-label, 2 × 2 | 4614 |
| 100 | 14 | 60 | 6 | Clinically relevant bleeding (major bleeding according to ISTH definition or clinically relevant non-major bleeding events) |
| ENTRUST-AF PCI | RCT open-label, 1:1 | 1506 |
| 100 | 5 | 52 | 12 | Clinically relevant major or non-major bleeding (according to ISTH criteria) |
| . | Design . | Patients (n) . | Treatment groups . | AF (%) . | Maximum time in TAT in DTA group (days) . | ACS (%) . | Follow-up (months) . | Primary outcome . |
|---|---|---|---|---|---|---|---|---|
| WOEST | RCT, open-label, 1:1 | 573 |
| 69 | <1 | 28 | 12 | Haemorrhagic events of any kind (according to the TIMI and GUSTO criteria) |
| ISAR-TRIPLE | RCT open-label, 1:1 | 614 |
| 84 | / | 32 | 9 | Composite endpoint of death, myocardial infarction, stent thrombosis, stroke or major bleeding |
| PIONEER-AF | RCT open-label, 1:1:1 | 2124 |
| 100 | 3 | 52 | 12 | Clinically relevant bleeding (major bleeding according to TIMI criteria, minor bleeding or bleeding that required medical intervention) |
| RE-DUAL PCI | RCT open-label, 1:1:1 | 2725 |
| 100 | 5 | 51 | 14 | Clinically relevant bleeding (major bleeding according to ISTH definition or clinically relevant non-major bleeding events) |
| AUGUSTUS | RCT open-label, 2 × 2 | 4614 |
| 100 | 14 | 60 | 6 | Clinically relevant bleeding (major bleeding according to ISTH definition or clinically relevant non-major bleeding events) |
| ENTRUST-AF PCI | RCT open-label, 1:1 | 1506 |
| 100 | 5 | 52 | 12 | Clinically relevant major or non-major bleeding (according to ISTH criteria) |
ACS, acute coronoary syndrome; AF, atrial fibrillation; bd, bis in die; GUSTO, Global Use of Streptokinase and t-PA for Occluded Coronary Arteries; ISTH, International Society on Thrombosis and Haemostasis; od, omni die; P2Y12i, P2Y12 platelet receptor inhibitors; RCT, randomized controlled trial; VKA, vitamin K antagonists.
Randomized clinical trials
The WOEST Study (What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing) was the first trial to compare a TAT regimen (Aspirin, Clopidogrel, and VKA) to a DAT regimen represented by clopidogrel and VKA.27 The study showed that a TAT regimen is associated with a significant increase in bleeding, compared to DAT. In the trial, ischaemic events such as death, heart attack, stroke, systemic embolism, revascularization, and stent thrombosis (17.6% vs. 11.1%, P = 0.025) increased significantly, despite the study being not sized to observe these differences. While acknowledging the value of the study, which first authorized a de-escalation of the antithrombotic regimen (from 3 to 2 antithrombotic drugs), the results of the WOEST Study should be analysed with caution. In fact, in the trial, not all patients had AF and/or ACS, the duration of TAT was prolonged, severe bleeding, in accordance with the TIMI and GUSTO definitions, were not significantly reduced in the DAT arm and the favourable results of DAT compared to TAT, especially in terms of reduction of mortality from all causes, were limited by low statistical power and not supported by the results of other clinical studies.
After the WOEST Study, the ISAR-TRIPLE (Triple Therapy in Patients on Oral Anticoagulation After Drug Eluting Stent Implantation) compared a 6-week TAT regimen to a 6-month one, demonstrating a reduction in the bleeding incidence in the 6-week group, compared to that in which the TAT lasted for 6 months.28
Subsequently, 4 randomized clinical trials were published that investigated the use of the 4 DOACs in a DAT regimen, mostly compared with VKA in TAT, in patients with AF undergoing PCI. Notably, the primary endpoint in the studies was represented only by haemorrhagic events, and therefore, the studies did not provide conclusive data on the efficacy of a DAT regimen in terms of ischaemic events. The PIONEER Study (oPen-Label, Randomized, Controlled, Multicenter Study ExplorIng TwO TreatmeNt StratEgiEs of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention) was the first to use a DOAC in DAT regimen and enrolled over 2100 patients with non-valvular AF undergoing PCI.6 The study showed that the association of a 15 mg/day dosage of rivaroxaban and a P2Y12 receptor inhibitor for 12 months or the association of a dosage of 2.5 mg × 2 of rivaroxaban for 1, 6, or 12 months, determined a significant reduction of the bleeding events, compared to the standard VKA and DAPT therapy for 1, 6, or 12 months. On the contrary, there were not significant differences in terms of ischaemic events in the three groups. Although PIONEER was the first randomized trial to evaluate the best combination of antithrombotic therapy with DOAC in patients with AF undergoing PCI, some limitations are evident. First, the definition of bleeding was not based on a standard classification but was considered as TIMI bleedings that required medical attention (meaning, among these, even cases in which repeating haematochemical exams were indicated). It is important to underline how, in this trial, only low dosages of rivaroxaban were used, not tested in pivotal trials in the prophylaxis of thromboembolic events in AF patients.
The RE-DUAL PCI (The open-label, Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran vs. Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percu-taneous Coronary Intervention) tested the approved dosages for AF of dabigatran (110 or 150 mg b.i.d.) in a DAT regimen, compared to a TAT regimen with VKA.7 The trial showed that the incidence of major and clinically relevant bleedings was significantly lower in DAT than in TAT: 15.4% in the 110 mg DAT group vs. 26.9% in the TAT group (P < 0.001 for non-inferiority; P < 0.001 for superiority), and 20.2% in the 150 mg DAT group vs. 25.7% in the TAT group (P < 0.001 for non-inferiority).7 In summary, the results of the three randomized trials mentioned so far (WOEST, PIONEER AF-PCI, and RE-DUAL PCI) have shown that a TAT regimen significantly increases bleeding events, apparently without conferring additional protection in preventing ischaemic events compared to the DAT regimen. The same trials, however, did not show whether the benefit, in haemorrhagic terms, was due to the number of antithrombotic drugs used (2 vs. 3) or to the type of anticoagulant administered (VKA vs. DOAC). The AUGUSTUS trial (An open-Label, 2 × 2 factorial, randomized controlled trial to evaluate the safety of apixaban vs. vitamin K antagonist and aspirin vs. placebo in patients with AF and acute coronary syndrome and/or percutaneous coronary intervention) sought to answer this question by comparing in about 5000 patients (the largest sample in this setting), with a 2 × 2 factorial design, apixaban (at the approved dosage for stroke prevention in AF) with VKA, in a DAT vs. a TAT regimen. The trial showed that apixaban was associated with a reduction in bleeding compared to VKA (10.5% vs. 14.5%, P < 0.001), and that the addition of aspirin resulted in an increase in bleeding events compared to placebo (16.1% vs. 9.0%, P < 0.001).8 In contrast, the incidence of ischaemic events did not increase in the DAT group compared to the TAT group with aspirin [7.3% vs. 6.5%, hazard ratio 1.12, 95% confidence interval (CI) 0.90–1.41].8 The latest study conducted with DOAC, the ENTRUST-AF PCI (Edoxaban Treatment vs. Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention), compared edoxaban (at the approved dosage for the prevention of stroke in AF) in ∼1500 patients in a DAT regimen with VKA in a TAT regimen, with a median PCI-randomization time of 45 h (interquartile range 22.2–76.2).9 The DAT group with edoxaban was no lower than the TAT group with VKA in terms of bleeding (major and clinically relevant) (17% vs. 20%; P = 0.0010 for non-inferiority; P = 0.1154 for superiority), with no differences in ischaemic events.9
A recent meta-analysis analysed the data of more than 10 000 patients with AF undergoing PCI and/or ACS included in the above-mentioned studies.29 The authors showed that a DAT regimen, compared to TAT, significantly reduced clinically relevant bleeding, but only a DAT that included DOACs was able to significantly reduce intracranial bleeding. In particular, DAT significantly reduced major bleeding (according to the classification of the International Society on Thrombosis and Haemostasis, ISTH) or clinically relevant bleeding (13.4% vs. 20.8%; P < 0.0001). In addition, DAT was associated with an incidence of major cardiovascular events, cardiovascular and all-cause mortality, and stroke, similar to that observed in TAT patients. However, the meta-analysis data showed an increase in cases of acute myocardial infarction and stent thrombosis at the limits of statistical significance. These risks appeared to be greater when patients on dabigatran 110 mg29 were separately placed in the DAT group. In contrast, clinical presentation (chronic vs. acute) did not appear to impact the efficacy/safety of the various antithrombotic strategies used.29 Another meta-analysis, including patients from the four randomized trials, showed that, at approximately 1 year of follow-up, a DAT regimen was associated with a reduced risk of major bleeding compared to a TAT (95% CI, −0.025 to −0.002).30 There were no conclusive results concerning any differences in all-cause mortality, cardiovascular mortality, myocardial infarction, stent thrombosis, and stroke.
Although the aforementioned clinical trials have provided a series of significant evidence that has led to a change in therapeutic strategies, it is emphasized that none of the four clinical trials had sufficient statistical power to demonstrate the effectiveness of a DAT regimen in terms of prevention of ischaemic events. In addition, aspirin withdrawal occurred at different times, the duration of follow-up ranged from 6 to 14 months, and TAT lasted from 1 to 12 months. Finally, in the absence of data on the procedural complexity of PCI and coronary anatomy, together with the low enrolment rate, it cannot be excluded that the populations in question were at low thrombotic risk and that the data emerging from the trials are not replicable in the same way in real-world patients.
Observational studies
In recent years, numerous observational studies have been published aimed at evaluating the type of antithrombotic strategy indicated for patients undergoing PCI who need a concomitant OAT. However, most of these studies are retrospective or derive from sub-analyses of large population registries, with all the known methodological limitations and related possible selection biases. The number of dedicated prospective observational studies are limited and are presented in Table 2.31–40 It can be observed from the analysis of these studies that the prevailing therapeutic strategy in clinical practice is TAT, even if the percentage of patients treated with DAPT alone is numerically well represented. DAT is only used in a minority of cases without any significant changes over time.
Prospective observational records on antithrombotic treatment in patients with atrial fibrillation undergoing coronary angioplasty with stent implantation
| Author/acronym . | Years . | . | Patients (n) . | TAT (%) . | DAT (%) . | DAPT (%) . | DOAC (%) . |
|---|---|---|---|---|---|---|---|
| Saratoff et al.31 | 2002–07 | Monocenter/Germany | 515 | 59 | 0 | 41 | 0 |
| STENTICO32 | 2005–06 | Multicentric/France | 359 | 100 | 0 | 0 | 0 |
| MUSICA33 | 2003–06 | Multicentric/Spain | 405 | 69 | 11 | 20 | 0 |
| AFCAS34 | 2008–10 | Multicentric/Europe | 914 | 74 | 8 | 18 | 0 |
| WARSTENT35 | 2008–10 | Multicentric/Italy | 401 | 85 | 5 | 10 | 0 |
| Sambola et al.36 | 2003–12 | Multicentric/Spain | 585 | 55 | 0 | 45 | 0 |
| Horie et al.37 | 2015–17 | Multicentric/Japan | 285 | 100 | 0 | 0 | 0 |
| CHUM AF STENT38 | 2010–19 | Multicentric/Canada | 561 | 44 | 8 | 47 | 32 |
| GRAPE AF39 | 2017–19 | Multicentric/Greece | 654 | 50 | 49 | 1 | 93 |
| MATADOR-PCI40 | 2018–19 | Multicentric/Italy | 588 | 65 | 9 | 26 | 62 |
| Author/acronym . | Years . | . | Patients (n) . | TAT (%) . | DAT (%) . | DAPT (%) . | DOAC (%) . |
|---|---|---|---|---|---|---|---|
| Saratoff et al.31 | 2002–07 | Monocenter/Germany | 515 | 59 | 0 | 41 | 0 |
| STENTICO32 | 2005–06 | Multicentric/France | 359 | 100 | 0 | 0 | 0 |
| MUSICA33 | 2003–06 | Multicentric/Spain | 405 | 69 | 11 | 20 | 0 |
| AFCAS34 | 2008–10 | Multicentric/Europe | 914 | 74 | 8 | 18 | 0 |
| WARSTENT35 | 2008–10 | Multicentric/Italy | 401 | 85 | 5 | 10 | 0 |
| Sambola et al.36 | 2003–12 | Multicentric/Spain | 585 | 55 | 0 | 45 | 0 |
| Horie et al.37 | 2015–17 | Multicentric/Japan | 285 | 100 | 0 | 0 | 0 |
| CHUM AF STENT38 | 2010–19 | Multicentric/Canada | 561 | 44 | 8 | 47 | 32 |
| GRAPE AF39 | 2017–19 | Multicentric/Greece | 654 | 50 | 49 | 1 | 93 |
| MATADOR-PCI40 | 2018–19 | Multicentric/Italy | 588 | 65 | 9 | 26 | 62 |
DAPT, dual antiplatelet therapy; DAT, dual antithrombotic therapy (anticoagulant + single antiplatelet); DOAC, direct anticoagulants; TAT, triple antithrombotic therapy (anticoagulant + dual antiplatelet therapy).
Prospective observational records on antithrombotic treatment in patients with atrial fibrillation undergoing coronary angioplasty with stent implantation
| Author/acronym . | Years . | . | Patients (n) . | TAT (%) . | DAT (%) . | DAPT (%) . | DOAC (%) . |
|---|---|---|---|---|---|---|---|
| Saratoff et al.31 | 2002–07 | Monocenter/Germany | 515 | 59 | 0 | 41 | 0 |
| STENTICO32 | 2005–06 | Multicentric/France | 359 | 100 | 0 | 0 | 0 |
| MUSICA33 | 2003–06 | Multicentric/Spain | 405 | 69 | 11 | 20 | 0 |
| AFCAS34 | 2008–10 | Multicentric/Europe | 914 | 74 | 8 | 18 | 0 |
| WARSTENT35 | 2008–10 | Multicentric/Italy | 401 | 85 | 5 | 10 | 0 |
| Sambola et al.36 | 2003–12 | Multicentric/Spain | 585 | 55 | 0 | 45 | 0 |
| Horie et al.37 | 2015–17 | Multicentric/Japan | 285 | 100 | 0 | 0 | 0 |
| CHUM AF STENT38 | 2010–19 | Multicentric/Canada | 561 | 44 | 8 | 47 | 32 |
| GRAPE AF39 | 2017–19 | Multicentric/Greece | 654 | 50 | 49 | 1 | 93 |
| MATADOR-PCI40 | 2018–19 | Multicentric/Italy | 588 | 65 | 9 | 26 | 62 |
| Author/acronym . | Years . | . | Patients (n) . | TAT (%) . | DAT (%) . | DAPT (%) . | DOAC (%) . |
|---|---|---|---|---|---|---|---|
| Saratoff et al.31 | 2002–07 | Monocenter/Germany | 515 | 59 | 0 | 41 | 0 |
| STENTICO32 | 2005–06 | Multicentric/France | 359 | 100 | 0 | 0 | 0 |
| MUSICA33 | 2003–06 | Multicentric/Spain | 405 | 69 | 11 | 20 | 0 |
| AFCAS34 | 2008–10 | Multicentric/Europe | 914 | 74 | 8 | 18 | 0 |
| WARSTENT35 | 2008–10 | Multicentric/Italy | 401 | 85 | 5 | 10 | 0 |
| Sambola et al.36 | 2003–12 | Multicentric/Spain | 585 | 55 | 0 | 45 | 0 |
| Horie et al.37 | 2015–17 | Multicentric/Japan | 285 | 100 | 0 | 0 | 0 |
| CHUM AF STENT38 | 2010–19 | Multicentric/Canada | 561 | 44 | 8 | 47 | 32 |
| GRAPE AF39 | 2017–19 | Multicentric/Greece | 654 | 50 | 49 | 1 | 93 |
| MATADOR-PCI40 | 2018–19 | Multicentric/Italy | 588 | 65 | 9 | 26 | 62 |
DAPT, dual antiplatelet therapy; DAT, dual antithrombotic therapy (anticoagulant + single antiplatelet); DOAC, direct anticoagulants; TAT, triple antithrombotic therapy (anticoagulant + dual antiplatelet therapy).
In Italy, it is possible to evaluate the temporal evolution of the antithrombotic strategy of patients with AF undergoing PCI, thanks to the results of the WAR-STENT (WARfarin and Coronary STENTing)35 and MATADOR-PCI (Management of Antithrombotic TherApy in Patients with Chronic or DevelOping AtRial Fibrillation During Hospitalization for PCI) studies.40 In the WAR-STENT Registry, conducted between 2008 and 2010, in the VKA era and in the absence of data on antithrombotic combinations, among the 401 patients recruited in 37 Italian centres, 85% had been discharged with TAT and only 5% of patients with DAT.35 Ten years later, between 2018 and 2019, in the MATADOR PCI, which recruited 598 patients with ACS and AF treated with stent implantation, TAT was prescribed in 65% of the cases with only 9% of the patients being discharged with DAT and 26% with DAPT.40 Similar percentages can be observed in other prospective studies, with the exception of the Greek GRAPE AF (Greek AntiplatElet Atrial Fibrillation),39 conducted between 2017 and 2019, in which the percentage of discharged patients with DAT was equal to 49%.
The data on the duration of TAT are extremely variable: in the WAR-STENT Registry35 and the Horie Registry,37 the median of treatment was 4 weeks, in the Sarafoff Registry31 of 12 weeks, in the AFCAS Registry (Management of patients with Atrial Fibrillation undergoing Coronary Artery Stenting)34 the average duration of TAT was 129 days and finally in the MATADOR-PCI40,41 at the follow-up of 6 months still 23.6% of the patients were taking a TAT (which corresponds to 40% of patients discharged with this therapeutic regimen).
Another aspect to highlight is the high use of DAPT, a therapeutic strategy that is not supported by current guidelines. For example, from an analysis of Danish registries on more than 12 000 patients enrolled, there were no differences in either CHA2DS2VASc [Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65–74 years, Sex category (female)] or HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score among patients treated with TAT or DAPT (who accounted for as many as 30% of the patients).42 A similar result was present in a data analysis from the TREAT-AF (Retrospective Evaluation and Assessment of Therapies in AF) a retrospective registry, conducted in the USA on over 4000 patients with AF, in which patients treated with DAPT (more than 50% of the total) had an average CHA2DS2VASc score of 3.6 compared with an average HAS-BLED of 2.5.43
In terms of prognosis, a major part of the studies show no significant differences among the various therapeutic regimens, but it should be taken into consideration that all the prospective published studies have rather small dimensions (<1000 patients), compare three different therapeutic regimens, and thus limit the reliability of the results due to the small sample analysed. More data on prognosis can be extracted from the retrospective analysis of the Swedish register SWEDEHEART.44 Over 7000 patients undergoing PCI and requiring OAT analysed between 2005 and 2012, the use of a TAT was associated with a significant increase in major bleeding, compared to DAT, without significant differences in terms of ischaemic events. However, it should be noted that of the over 7000 patients recruited, only 16% received a TAT and 9% a DAT.
Even the ‘real life’ data comparing the various types of OAT used, especially between VKA and DOACs, are very limited in terms of prognosis.45 The only direct comparison data comes from a retrospective analysis of Danish administrative data conducted between 2011 and 2017,46 which included over 3000 patients with myocardial infarction and/or PCI who needed concomitant OAT. The use of DOAC in the context of a TAT was associated with a significant reduction in the major bleeding risk, compared to a TAT with VKA (4.89% vs. 9.39%) with no difference in mortality (8.79% vs. 10.48%, respectively). On the other hand, when a DAT was used, there were no significant differences between DOAC and VKA, both in terms of mortality (15.5% vs. 14.6%, respectively) and bleedings (6.2% vs. 601%).
In the near future, updated observational data may come from a new Italian multicentre study conducted on patients on anticoagulant therapy undergoing stent implantation (PERSEO, ClinicalTrials.gov Identifier: NCT03392948). The study, which represents the largest observational study designed so far (a recruitment of about 1500 patients is expected), with a 1-year clinical follow-up, will provide data on the current Italian prescribing reality and also prognostic information on the various therapeutic strategies used.
Guidelines and consensus documents
The most recent indications on the management of antithrombotic therapy reported by the 2020 ESC Guidelines on the management of AF and ACS are based on the results of randomized clinical trials.6–10,47–49 The message that emerges from the two recent ESC documents is the need to customize antithrombotic therapy (TAT or DAT) based on the ischaemic and haemorrhagic risk profile of the individual patient, both in terms of the composition and duration of the therapeutic regimen. The guidelines state that, where not contraindicated, DOACs should be preferred over VKAs, with a possible dose reduction in accordance with the label’ criteria. If there are contraindications to the use of DOACs, the administration of VKA should be calibrated to maintain an INR range between 2.0 and 2.5.
Antiplatelet therapy should be prescribed by evaluating the patient’s clinical presentation (acute or chronic setting). Regarding the choice of the duration, type, and number of antiplatelet agents to be administered, the guidelines once again underline the importance of using the appropriate scores to identify subjects considered at high risk of bleeding complications and/or at high risk of thrombotic complications. A HAS-BLED ≥3 identifies patients considered to be at high risk of bleeding, while the ischaemic risk assessment makes use of the presence of specific indicators of possible thrombotic complications that include both particular comorbidities of the patient and factors related to the coronary revascularization procedure itself (Table 3). According to the guidelines, AF and Chronic Coronary Syndromes (CCS) patients considered to be at high bleeding risk and at low risk of stent-related complications, TAT is prescribed for approximately one week with subsequent interruption of aspirin and continuation of clopidogrel and DOAC/VKA (DAT) for at least 12 months (Class IA indication). Where there are elements of greater risk for intracoronary thrombotic complications, it is instead possible to evaluate the extension of the duration of TAT for a longer period, which, however, should not be more than a month (indication of Class IIa C).
| High ischaemic risk . | Moderate ischaemic risk . |
|---|---|
| Complex coronary artery disease and at least one criterion | Non-complex coronary artery disease and at least one criterion |
| Risk enhancers | |
| Diabetes mellitus in therapy | Diabetes mellitus in therapy |
| Previous ACS/recurrent myocardial infarction | Previous ACS/recurrent myocardial infarction |
| Multi-vessel coronary disease | Polydistrict artery disease (coronary artery disease plus peripheral artery disease) |
| Polydistrict artery disease (coronary artery disease plus peripheral artery disease) | Renal impairment (eGFR 15–59 mL/min) |
| Coronary artery disease at a young age (<45 years) or rapidly progressive (appearance of new lesions within 2 years) | |
| Renal impairment (eGFR 15–59 mL/min) | |
| Concomitant systemic inflammatory disease (e.g. HIV, LES, chronic arthritis) | |
| Procedural technical aspects | |
| Implantation of at least three stents | |
| Treatment of at least three lesions | |
| Total length of stent >60 mm | |
| History of complex revascularization (common trunk, bifurcation stenting with ≥2 implanted stents, chronic total occlusions, stents on last patent vessel) | |
| History of intrastent thrombosis in antiplatelet therapy | |
| High ischaemic risk . | Moderate ischaemic risk . |
|---|---|
| Complex coronary artery disease and at least one criterion | Non-complex coronary artery disease and at least one criterion |
| Risk enhancers | |
| Diabetes mellitus in therapy | Diabetes mellitus in therapy |
| Previous ACS/recurrent myocardial infarction | Previous ACS/recurrent myocardial infarction |
| Multi-vessel coronary disease | Polydistrict artery disease (coronary artery disease plus peripheral artery disease) |
| Polydistrict artery disease (coronary artery disease plus peripheral artery disease) | Renal impairment (eGFR 15–59 mL/min) |
| Coronary artery disease at a young age (<45 years) or rapidly progressive (appearance of new lesions within 2 years) | |
| Renal impairment (eGFR 15–59 mL/min) | |
| Concomitant systemic inflammatory disease (e.g. HIV, LES, chronic arthritis) | |
| Procedural technical aspects | |
| Implantation of at least three stents | |
| Treatment of at least three lesions | |
| Total length of stent >60 mm | |
| History of complex revascularization (common trunk, bifurcation stenting with ≥2 implanted stents, chronic total occlusions, stents on last patent vessel) | |
| History of intrastent thrombosis in antiplatelet therapy | |
The stratification of patients in high or moderate risk of coronary artery disease is based on the individual assessment of the clinician, who is aware of the patient’s cardiovascular history and/or coronary anatomy.
ACS, acute coronary syndrome; eGFR, glomerular filtration rate; HIV, Human immunodeficiency virus; LES, systemic lupus erythematosus.
| High ischaemic risk . | Moderate ischaemic risk . |
|---|---|
| Complex coronary artery disease and at least one criterion | Non-complex coronary artery disease and at least one criterion |
| Risk enhancers | |
| Diabetes mellitus in therapy | Diabetes mellitus in therapy |
| Previous ACS/recurrent myocardial infarction | Previous ACS/recurrent myocardial infarction |
| Multi-vessel coronary disease | Polydistrict artery disease (coronary artery disease plus peripheral artery disease) |
| Polydistrict artery disease (coronary artery disease plus peripheral artery disease) | Renal impairment (eGFR 15–59 mL/min) |
| Coronary artery disease at a young age (<45 years) or rapidly progressive (appearance of new lesions within 2 years) | |
| Renal impairment (eGFR 15–59 mL/min) | |
| Concomitant systemic inflammatory disease (e.g. HIV, LES, chronic arthritis) | |
| Procedural technical aspects | |
| Implantation of at least three stents | |
| Treatment of at least three lesions | |
| Total length of stent >60 mm | |
| History of complex revascularization (common trunk, bifurcation stenting with ≥2 implanted stents, chronic total occlusions, stents on last patent vessel) | |
| History of intrastent thrombosis in antiplatelet therapy | |
| High ischaemic risk . | Moderate ischaemic risk . |
|---|---|
| Complex coronary artery disease and at least one criterion | Non-complex coronary artery disease and at least one criterion |
| Risk enhancers | |
| Diabetes mellitus in therapy | Diabetes mellitus in therapy |
| Previous ACS/recurrent myocardial infarction | Previous ACS/recurrent myocardial infarction |
| Multi-vessel coronary disease | Polydistrict artery disease (coronary artery disease plus peripheral artery disease) |
| Polydistrict artery disease (coronary artery disease plus peripheral artery disease) | Renal impairment (eGFR 15–59 mL/min) |
| Coronary artery disease at a young age (<45 years) or rapidly progressive (appearance of new lesions within 2 years) | |
| Renal impairment (eGFR 15–59 mL/min) | |
| Concomitant systemic inflammatory disease (e.g. HIV, LES, chronic arthritis) | |
| Procedural technical aspects | |
| Implantation of at least three stents | |
| Treatment of at least three lesions | |
| Total length of stent >60 mm | |
| History of complex revascularization (common trunk, bifurcation stenting with ≥2 implanted stents, chronic total occlusions, stents on last patent vessel) | |
| History of intrastent thrombosis in antiplatelet therapy | |
The stratification of patients in high or moderate risk of coronary artery disease is based on the individual assessment of the clinician, who is aware of the patient’s cardiovascular history and/or coronary anatomy.
ACS, acute coronary syndrome; eGFR, glomerular filtration rate; HIV, Human immunodeficiency virus; LES, systemic lupus erythematosus.
In the event that a patient is already on antiplatelet therapy and only subsequently develops AF, the indications may vary. In the presence of a patient treated with antiplatelet therapy and confirmed AF, as reported in the expert consensus document of the American College of Cardiology (ACC),51 the first thing to do, after evaluating whether there is an indication to administer an OAT and the haemorrhagic and ischaemic/thrombotic risk, is to evaluate the indication for the continuation of the current antiplatelet therapy. In patients treated with aspirin or clopidogrel in primary prevention or with a CCS, without previous acute events or not treated with revascularization procedures, it is possible to suspend antiplatelet aggregation and continue with the OAT only when AF is detected. In patients who have been treated with PCI and stent implantation for a CCS, it is advisable to suspend DAPT and continue with a DAT if AF occurs within 6 months of the procedure or discontinue antiplatelet aggregation and administer OAT only if AF appears over 6 months from the PCI. For patients treated with PCI for ACS with indication to a DAPT for 12 months, the indication is to discontinue aspirin and continue a DAT for 1 year from the index event.52
Definition of the risk profiles
Thromboembolic risk
AF is generally associated with a risk of stroke five times higher than patients in sinus rhythm. This risk, however, is not homogeneously distributed, but is related to the presence of specific risk factors or risk modifiers.53,54
Commonly used thromboembolic risk factors are included in the CHA2DS2-VASc score.54 This score is able to accurately identify patients at low risk of stroke (less than 1% per year), with CHA2DS2-VASc 0 (males) or 1 (females), who do not need OAT. Instead, it has a lower predictive value in high-risk patients, similarly to other scores based on clinical risk factors. In addition to CHA2DS2-VASc, other more complex clinical scores were studied, but the improvement in stroke predictability, while significant, was modest55–57 (Table 4).
| TE risk score . | Variables used . | Score . | TE risk classes . | C-index (95% IC) . | Practical use (+) . |
|---|---|---|---|---|---|
| ATRIA | Age, HR, DM, HF, Hypertension, proteinuria, eGFR <45 mL/min/1.73 m2 or ESRD | From 0 to 12 in absence of previous stroke, from 0 to 15 in absence of previous stroke |
| 0.73 (0.71–0.75)a | ++ |
| ABC-stroke | Age, previous stroke or TIA, NT-proBNP, hs-TnT | Nomograms | 0.67 (0.65–0.70)b | ++ | |
| Age, female, SBP, vascular disorders, previous bleeding, renal failure, use of OAC | Electronic calculation system | 0.75 (0.73–0.77)c | + | |
| CHA2DS2-VASc | HF, hypertension, age, DM, Previous stroke, vasculopathy | From 0 to 9 |
| ++++ |
| TE risk score . | Variables used . | Score . | TE risk classes . | C-index (95% IC) . | Practical use (+) . |
|---|---|---|---|---|---|
| ATRIA | Age, HR, DM, HF, Hypertension, proteinuria, eGFR <45 mL/min/1.73 m2 or ESRD | From 0 to 12 in absence of previous stroke, from 0 to 15 in absence of previous stroke |
| 0.73 (0.71–0.75)a | ++ |
| ABC-stroke | Age, previous stroke or TIA, NT-proBNP, hs-TnT | Nomograms | 0.67 (0.65–0.70)b | ++ | |
| Age, female, SBP, vascular disorders, previous bleeding, renal failure, use of OAC | Electronic calculation system | 0.75 (0.73–0.77)c | + | |
| CHA2DS2-VASc | HF, hypertension, age, DM, Previous stroke, vasculopathy | From 0 to 9 |
| ++++ |
DM, diabete mellitus; ESRD, end-stage renal disease; HF, heart failure; HR, heart rate; hs-TnT, high-sensitivity troponin T; NT-proBNP, terminal amino fragment of propeptide type B; OAC, oral anticoagulants; SBP, systolic blood pressure; TE, thromboembolic.
C-index for TE events at 1 year.
C-index for stroke/systemic embolic events at 1 year; the study population was all on an anticoagulant regimen.
C-index for all-cause mortality at 1 year.
| TE risk score . | Variables used . | Score . | TE risk classes . | C-index (95% IC) . | Practical use (+) . |
|---|---|---|---|---|---|
| ATRIA | Age, HR, DM, HF, Hypertension, proteinuria, eGFR <45 mL/min/1.73 m2 or ESRD | From 0 to 12 in absence of previous stroke, from 0 to 15 in absence of previous stroke |
| 0.73 (0.71–0.75)a | ++ |
| ABC-stroke | Age, previous stroke or TIA, NT-proBNP, hs-TnT | Nomograms | 0.67 (0.65–0.70)b | ++ | |
| Age, female, SBP, vascular disorders, previous bleeding, renal failure, use of OAC | Electronic calculation system | 0.75 (0.73–0.77)c | + | |
| CHA2DS2-VASc | HF, hypertension, age, DM, Previous stroke, vasculopathy | From 0 to 9 |
| ++++ |
| TE risk score . | Variables used . | Score . | TE risk classes . | C-index (95% IC) . | Practical use (+) . |
|---|---|---|---|---|---|
| ATRIA | Age, HR, DM, HF, Hypertension, proteinuria, eGFR <45 mL/min/1.73 m2 or ESRD | From 0 to 12 in absence of previous stroke, from 0 to 15 in absence of previous stroke |
| 0.73 (0.71–0.75)a | ++ |
| ABC-stroke | Age, previous stroke or TIA, NT-proBNP, hs-TnT | Nomograms | 0.67 (0.65–0.70)b | ++ | |
| Age, female, SBP, vascular disorders, previous bleeding, renal failure, use of OAC | Electronic calculation system | 0.75 (0.73–0.77)c | + | |
| CHA2DS2-VASc | HF, hypertension, age, DM, Previous stroke, vasculopathy | From 0 to 9 |
| ++++ |
DM, diabete mellitus; ESRD, end-stage renal disease; HF, heart failure; HR, heart rate; hs-TnT, high-sensitivity troponin T; NT-proBNP, terminal amino fragment of propeptide type B; OAC, oral anticoagulants; SBP, systolic blood pressure; TE, thromboembolic.
C-index for TE events at 1 year.
C-index for stroke/systemic embolic events at 1 year; the study population was all on an anticoagulant regimen.
C-index for all-cause mortality at 1 year.
Importantly, thromboembolic risk factors are dynamic and need to be regularly re-evaluated during follow-up. More than 15% of patients with a low initial risk manifest at least one stroke risk factor within 1 year. Those who change their risk score are more likely to develop a stroke.58
Atherothrombotic risk
According to the guidelines, patients with coronary disease can be stratified into two different ischaemic risk groups: high and moderate risk, defined according to an individual clinical judgement based on the patient's cardiovascular medical history and/or coronary anatomy and procedural factors10 (Table 3). In the context of AF patients undergoing PCI, innovative risk scores have recently been evaluated, but they need validation in large population studies.59,60
However, in light of the above evidence, the risk of acute/subacute stent thrombosis (≤30 days after PCI) should also be taken into account in patients with AF undergoing PCI. Numerous studies have identified that the following procedural risk factors are associated with an increased risk of acute stent thrombosis61: final TIMI grade flow 0/1, residual dissection, intracoronary residual thrombosis, inadequate stent expansion, and extensive stent overlapping. In these cases, protection with effective antithrombotic drugs for the first few weeks after implantation is of pivotal importance, regardless of the patient's bleeding risk (except for the presence of major or life-threatening bleeding).
Haemorrhagic risk
Over the years, different scores for the estimation of haemorrhagic risk have been validated in patients with ACS and/or subjected to PCI, such as CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) and ACUITY (Acute Catheterization and Urgent Intervention Triage strategY), which despite having a good predictive accuracy, have never been validated in patients taking OAT.62,63
Similarly, the HAS-BLED score, validated in AF patients, has not been validated in ACS or in CCS treated with PCI,64,65 but only tested in small observational studies.
Recently, the ARC-HBR (Academic Research Consortium high bleeding risk) score (Table 5) has been introduced as an alternative to these scores. It is a pragmatic approach, evaluated in independent contemporary cohorts of patients at high haemorrhagic risk in the PCI setting. Patients must have at least one higher or two minor risk criteria to be considered at high risk of bleeding; the coexistence of multiple ARC-HBR factors has an additional prognostic impact.66,67 The objective of this score is to provide a more accurate risk stratification, allowing, in each case, the identification of the most appropriate combination of antiplatelets and oral anticoagulants.
| Major criteria . | Minor criteria . |
|---|---|
| Age >75 years | |
| Anticipated need for long-term anticoagulant therapya | |
| Severe or terminal chronic renal impairment (eVFR < 30 mL/min) | Moderate chronic renal impairment (eVFR 30–59 mL/min) |
| Haemoglobin <11 g/dL | Haemoglobin 11–12.9 g/dL in males or 11–11.9 in females |
| Spontaneous bleeding that has required transfusion during hospitalization in the past 6 months or at any time, if recurrent | Spontaneous bleeding that has required hospitalization or transfusions in the last 12 months that do not satisfy the major criteria |
| Moderate or severe basalb platelet penia (platelet count < 100 × 109/L) | |
| Haemorrhagic diathesis | |
| Liver cirrhosis with portal hypertension | |
| Long-term use of corticosteroids or NSAID | |
| Active neoplasmc (with the exception of skin neoplasms other than melanoma) in the last 12 months | |
| Previous spontaneous intraparenchymal haemorrhage (at any time) Traumatic intraparenchymal haemorrhage (in the last 12 months) | Any ischaemic stroke in any period of time that does not meet the major criteria |
| Presence of cerebral AVM | |
| Ischaemic stroked of severe or major degree in the last 6 months | |
| Non-deferrable major surgery during DAPT | |
| Recent major surgery or major trauma in the last 30 days |
| Major criteria . | Minor criteria . |
|---|---|
| Age >75 years | |
| Anticipated need for long-term anticoagulant therapya | |
| Severe or terminal chronic renal impairment (eVFR < 30 mL/min) | Moderate chronic renal impairment (eVFR 30–59 mL/min) |
| Haemoglobin <11 g/dL | Haemoglobin 11–12.9 g/dL in males or 11–11.9 in females |
| Spontaneous bleeding that has required transfusion during hospitalization in the past 6 months or at any time, if recurrent | Spontaneous bleeding that has required hospitalization or transfusions in the last 12 months that do not satisfy the major criteria |
| Moderate or severe basalb platelet penia (platelet count < 100 × 109/L) | |
| Haemorrhagic diathesis | |
| Liver cirrhosis with portal hypertension | |
| Long-term use of corticosteroids or NSAID | |
| Active neoplasmc (with the exception of skin neoplasms other than melanoma) in the last 12 months | |
| Previous spontaneous intraparenchymal haemorrhage (at any time) Traumatic intraparenchymal haemorrhage (in the last 12 months) | Any ischaemic stroke in any period of time that does not meet the major criteria |
| Presence of cerebral AVM | |
| Ischaemic stroked of severe or major degree in the last 6 months | |
| Non-deferrable major surgery during DAPT | |
| Recent major surgery or major trauma in the last 30 days |
DAPT, dual antiplatelet therapy; eVFR, estimated glomerular filtration rate; HBR, high haemorrhagic risk; MAV, arteriovenous malformation; NSAID, non-steroidal anti-inflammatory drugs.
Except for vascular protection doses.
Basal thrombocytopaenia is defined as thrombocytopaenua before coronary angioplasty.
Active neoplasm is defined as such if diagnosed in the last 12 months and/or during therapy (including surgery, chemotherapy, or radiotherapy).
On the basis of the National Institutes of Health Stroke Scale ≥5.
| Major criteria . | Minor criteria . |
|---|---|
| Age >75 years | |
| Anticipated need for long-term anticoagulant therapya | |
| Severe or terminal chronic renal impairment (eVFR < 30 mL/min) | Moderate chronic renal impairment (eVFR 30–59 mL/min) |
| Haemoglobin <11 g/dL | Haemoglobin 11–12.9 g/dL in males or 11–11.9 in females |
| Spontaneous bleeding that has required transfusion during hospitalization in the past 6 months or at any time, if recurrent | Spontaneous bleeding that has required hospitalization or transfusions in the last 12 months that do not satisfy the major criteria |
| Moderate or severe basalb platelet penia (platelet count < 100 × 109/L) | |
| Haemorrhagic diathesis | |
| Liver cirrhosis with portal hypertension | |
| Long-term use of corticosteroids or NSAID | |
| Active neoplasmc (with the exception of skin neoplasms other than melanoma) in the last 12 months | |
| Previous spontaneous intraparenchymal haemorrhage (at any time) Traumatic intraparenchymal haemorrhage (in the last 12 months) | Any ischaemic stroke in any period of time that does not meet the major criteria |
| Presence of cerebral AVM | |
| Ischaemic stroked of severe or major degree in the last 6 months | |
| Non-deferrable major surgery during DAPT | |
| Recent major surgery or major trauma in the last 30 days |
| Major criteria . | Minor criteria . |
|---|---|
| Age >75 years | |
| Anticipated need for long-term anticoagulant therapya | |
| Severe or terminal chronic renal impairment (eVFR < 30 mL/min) | Moderate chronic renal impairment (eVFR 30–59 mL/min) |
| Haemoglobin <11 g/dL | Haemoglobin 11–12.9 g/dL in males or 11–11.9 in females |
| Spontaneous bleeding that has required transfusion during hospitalization in the past 6 months or at any time, if recurrent | Spontaneous bleeding that has required hospitalization or transfusions in the last 12 months that do not satisfy the major criteria |
| Moderate or severe basalb platelet penia (platelet count < 100 × 109/L) | |
| Haemorrhagic diathesis | |
| Liver cirrhosis with portal hypertension | |
| Long-term use of corticosteroids or NSAID | |
| Active neoplasmc (with the exception of skin neoplasms other than melanoma) in the last 12 months | |
| Previous spontaneous intraparenchymal haemorrhage (at any time) Traumatic intraparenchymal haemorrhage (in the last 12 months) | Any ischaemic stroke in any period of time that does not meet the major criteria |
| Presence of cerebral AVM | |
| Ischaemic stroked of severe or major degree in the last 6 months | |
| Non-deferrable major surgery during DAPT | |
| Recent major surgery or major trauma in the last 30 days |
DAPT, dual antiplatelet therapy; eVFR, estimated glomerular filtration rate; HBR, high haemorrhagic risk; MAV, arteriovenous malformation; NSAID, non-steroidal anti-inflammatory drugs.
Except for vascular protection doses.
Basal thrombocytopaenia is defined as thrombocytopaenua before coronary angioplasty.
Active neoplasm is defined as such if diagnosed in the last 12 months and/or during therapy (including surgery, chemotherapy, or radiotherapy).
On the basis of the National Institutes of Health Stroke Scale ≥5.
Decision-making algorithm on antithrombotic therapy in AF patients undergoing PCI and/or ACS
In light of the evidence presented so far, a decision-making algorithm is proposed for the choice of the antithrombotic strategy to be prescribed at the time of hospital discharge of an AF patient and indication for OAT, undergoing PCI and/or ACS (with or without PCI) (Figure 4). The proposed drug combinations are formulated with the various DOACs, as they are believed to be preferable to VKAs. This does not exclude that, where necessary or clinically indicated, VKA may be used instead of DOACs with an INR between 2.0 and 2.5. It must be specified that DOACs should be used at full dosage. Dabigatran alone, in the case of DAT, can be used at both high and low dosages, as tested in the REDUAL-PCI study.7 The dosage of the other DOACs should only be reduced if the criteria set out in the label are met.
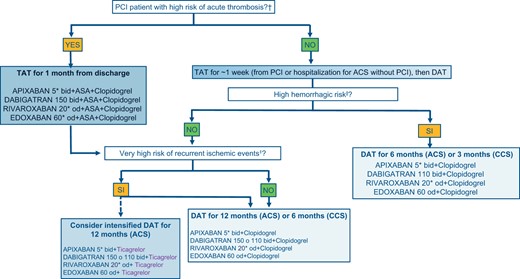
Decision-making algorithm on antithrombotic therapy to be applied at the time of discharge of AF patients undergoing PCI. N.B. It is possible to replace clopidogrel or ticagrelor with aspirin in case of intolerance, contraindications or side effects. ACS, acute coronary syndrome; CCS, chronic coronary syndrome; DAT, double antithrombotic therapy (1 antiplatelet + 1 anticoagulant); PCI, coronary angioplasty; TAT, triple antithrombotic therapy (2 antiplatelet + 1 anticoagulant). aFinal TIMI flow 0/1, residual dissection, residual intracoronary thrombosis, or subtraction angiography images, inadequate stent expansion or rupture; see Table 5 criteria, excluding long-term OA from the assessment of the major criteria. To be considered at high bleeding risk, patients must have at least one higher risk criterion or at least two minors; bSee criteria in Table 3; cReduce the dosage according to the instructions in the package leaflet.
In general, the period of TAT should not exceed 1 week after PCI or hospitalization for ACS not subject to revascularization, with the exception of patients at high risk of acute stent thrombosis. This recommendation is based on trials that have documented an increase in bleeding events, without any benefit in terms of reduction of ischaemic events, after 7 days from randomization to the TAT arm.68,69 For this reason, the first evaluation to be considered is related to the risk of acute thrombosis, in order to identify patients who can benefit most from the extended TAT up to a month. Patients who, after PCI, do not present procedural characteristics related to high acute thrombosis risk, which to date are the absolute majority, and patients with ACS not subject to coronary revascularization during the index event, should be subjected to a haemorrhagic risk assessment with a modern, universal and validated score, such as ARC-HBR. Obviously, in this context, the long-term OAT (major criterion) for the calculation of the severity of the risk should not be considered, as specifically, all patients with AF are subjected to OAT for an indefinite period.70 If the patient has the characteristics of high haemorrhagic risk, the use of DAT with clopidogrel is suggested to be reduced to 6 months for patients with ACS and to 3 months for patients with CCS, except for patients experiencing haemorrhagic events (in this case the antiplatelet or DAT should be discontinued early depending on the severity and reversibility of the haemorrhagic event). Obviously, in the cases of patients with prohibitive bleeding risk, it seems reasonable to use OAT only at hospital discharge.
In patients who do not present high-risk haemorrhagic characteristics, it is suggested to evaluate the presence of any recurrent high-risk ischaemic event criteria. In this case, it is even possible to use an intensified DAT with ticagrelor in association with full-dose DOACs. It should be noted that ticagrelor has been used in almost all of the studies with AF and PCI/ACS patients, although this has only been used in the context of the DAT regimen and in a minority of cases (about 12% in REDUAL-PCI7 and AUGUSTUS8) Although the subgroups are limited, no interaction has been observed in favour (reduction of ischaemic events) or against (increase in haemorrhagic events) of this association with respect to clopidogrel in the various sub-analyses published so far.71 However, it should be noted that a recent synthesis of the evidence available in this context compared an antithrombotic strategy with potent P2Y12 inhibitors (ticagrelor or prasugrel) to clopidogrel, documented an increase in clinically relevant bleeding events.72 For this reason, the DOAC/ticagrelor combination should only be reserved to specific selected cases of AF and ACS patients at high risk of recurrent ischaemic events and at low haemorrhagic risk. The use of DAT for 12 months in the case of ACS and 6 months for CCS is suggested in patients with no high bleeding risk criteria and with a high or moderate risk of recurrent ischaemic events. Aspirin may be preferred to ticagrelor or clopidogrel, in case of intolerance, contraindications, or side effects, in all the cases where a DAT regimen is adopted.
Specific subgroups of patients
Beyond the indications suggested above, there are subgroups of patients that deserve separate treatment and specific recommendations.
Severe/terminal renal impairment
In the four randomized clinical trials that led to the approval of the DOACs,73,-76 severe renal impairment, corresponding to an estimated glomerular filtration rate (eGFR) <30 mL/min, represented an exclusion criterion for enrolment and therefore no data are available for this population, nor for those with end-stage renal impairment (i.e. with eGFR <15 mL/min). In the observational, prospective, multicentre AFCAS Registry,77 conducted in the period prior to the introduction of DOACs and therefore with VKAs, 975 patients were enrolled, of which 781 (80%) the eGFR value was available. The eGFR value was <15 mL/min in only 7 patients who were analysed together with those with an eGFR value of <30 mL/min (4% of the total population).77 Compared to the eGFR >90 mL/min population, the eGFR subgroup <30 mL/min showed no significant differences in the antithrombotic therapy prescribed at discharge, i.e. TAT with VKA, aspirin and clopidogrel, DAT with VKA and clopidogrel or DAPT with aspirin and clopidogrel, nor any relative to the prescribed duration of clopidogrel administration (∼6 months).69 Compared to the eGFR >90 mL/min population, the eGFR <30 mL/min subgroup showed a major significant incidence at 12 months of (i) major adverse cardio-cerebrovascular events, including all-cause mortality, myocardial infarction, urgent re-revascularization, stent thrombosis, transient ischaemic attack, stroke, and systemic embolism, (ii) mortality, and (iii) total bleeding (BARC 1–5). Major bleeding (BARC > 2) did not show a significantly different incidence in the two eGFR groups of >90 mL/min and <30 mL/min, respectively, although in the latter they were twice as frequent.77
Although not directly applicable to patients with eGFR <15 mL/min (not adequately represented), the AFCAS Registry data77 support the established notion of an increased ischaemic and haemorrhagic risk in these patients, in whom VKA are today only oral anticoagulants78 that can be prescribed. However, the OAT benefit/risk ratio for stroke prevention in patients with AF and end-stage renal impairment is uncertain, to the point that the choice whether to anticoagulate should be carefully individualized and the oral anticoagulant possibly omitted.78
In view of this and the increased bleeding risk invariably associated with TAT, especially at the moment of its initiation,79 VKA should preferably be omitted and DAPT alone continued in patients with eGFR <15 mL/min as well as in patients at high risk of stroke.
Ventricular thrombosis
In view of the current use of DOACs only for the prevention of thromboembolism in AF and venous thromboembolic disease (VTE), in the presence of left ventricular thrombosis (LVT), which can be typically found in extended anterior infarction, the only oral anticoagulants to be used are VKAs with INR 2.0–3.0 for 3–6 months.80 In case of concomitant PCI, a brief TAT with VKA (INR target 2.0–3.0) and DAPT with aspirin and clopidogrel followed by DAT with AVK and clopidogrel for 3–12 months (in relation to individual bleeding risk) should therefore be established (1–4 weeks). At the end of which, upon documentation of successful resolution of the LVT, the transition from VKA to DOAC for long-term oral anticoagulation should be considered.81
Some observational series and small monocentric retrospective studies have recently reported similar efficacy and safety of VKA and DOAC in patients with LVT.82 In contrast, a multicentre retrospective cohort study of 514 patients showed a significant increase in the risk of stroke or systemic embolism with DOAC (44% of the total cohort, distributed as follows: 76% apixaban, 25% rivaroxaban, and 5% dabigatran) compared to VKA.83 However, there are numerous limitations emphasized by the authors themselves, such as the retrospective nature of the study and the absence of centralization in the evaluation of echocardiographic images, as well as the unavailability of data on DOAC dosages taken and adherence to treatment, which can weaken the observed results.83
To date, the use of DOACs can be considered, although with off-label indication, when associated with ischaemic stroke or transient ischaemic attack in the context of acute myocardial infarction associated with LVT or abnormalities of anterior or apical wall movement with a FEVS <40% in patients who are intolerant to VKA therapy due to non-haemorrhagic adverse events.84 This indication should also be considered in AF patients receiving DOAC prior to the development of LVT in whom a temporary replacement of the DOAC with VKA should generally be carried out until the resolution of the LVT itself.
New atrial fibrillation onset
Approximately 10% of ACS patients develop AF during the index event.85 In the MATADOR-PCI Registry,40 DOACs were the most frequently used OAT, with no difference to whether AF was pre-existing or newly developed. In contrast, in a monocentric registry conducted in Vienna, new onset AF was associated with a significant less use of TAT compared to DAT, but with a significantly better prognostic impact.85,86 However, it seems appropriate to suggest that an initial and short-term TAT should be recommended in all cases of new AF onset during ACS, if there is indication of OAT and the documented duration of the arrhythmia is >24 h.
Thrombocytopenia
Thrombocytopenia, defined as a platelet count of <150 000/mL, may occur in 5–13% of cases87 of ACS patients. It may already be present in baseline conditions (i.e. in the context of a neoplastic involvement of the bone marrow), or as a result of myelotoxic action exerted by the neoplasm and/or antineoplastic therapies, or be secondary to the use of drugs, such as following the establishment of therapy with heparin or glycoprotein IIb/IIIa inhibitors or in the context of PCI.87,88 Compared to its absence, the presence of thrombocytopenia, both basal and secondary, in PCI and/or ACS is associated with unfavourable clinical outcomes, both in haemorrhagic and ischaemic terms, with an increase in total mortality.89,90 In the absence of adequate and solid evidence, current suggestions to limit bleeding risk in AF patients undergoing PCI and significant thrombocytopenia (platelet count <100 000/mL) firstly include the identification and correction, even with onco-haematological counselling, of the potential reversible causes of thrombocytopenia.87 In case of persistent <50 000/mL platelet count and/or ongoing bleeding, all antithrombotic drugs should be interrupted and PCI avoided.87,88 On the other hand, when thrombocytopenia is mild-moderate (platelet count 50 000–150 000/mL) the same antithrombotic strategies used in general should be applied (TAT with DOAC, aspirin, and clopidogrel for a few days and then DAT with DOAC and clopidogrel). In the presence of a low-to-moderate stroke risk of—as exemplified by a CHA2DS2-VASc score <2 in males and <3 in females—temporary interruption (1–6 months) of TAT and its replacement with DAPT could be considered, to be followed by DAT with DOAC and clopidogrel (within the first 6 months) or monotherapy with DOAC (after 6 months), in view of a potentially higher significant clinical benefit.91
Venous thromboembolism
In the context of AF patients undergoing PCI, the possibility of simultaneous development of VTE should be considered, which in turn represents an indication for OAT. There are no studies dedicated to this specific clinical context, nor to that of patients with VTE alone undergoing PCI and who therefore require combined anticoagulant and antiplatelet therapy. In the studies, mostly observational, that were examined with OAT indications undergoing PCI, the VTE prevalence was 2–17% (average 10%) compared to 22–85% (average 61%) of AF.51,92
It seems reasonable to recommend that when VTE develops in the context of a PCI in an AF patient (for whom there is already an indication to OAT for an unlimited period of time), the antithrombotic strategies to be applied are the general ones, namely an initial and short TAT with DOAC, aspirin, and clopidogrel, followed by DAT. However, it should be considered that in VTE the initiation of OAT with apixaban and rivaroxaban involves a loading dose represented by 10 mg every 12 h for a week for apixaban and 15 mg every 12 h for 21 days for rivaroxaban.93 In the absence of specific data on the use of the loading dose when administered in TAT, it appears prudent, in this context, to favour dabigatran and edoxaban. In VTE patients, these should be administered at standard doses of 150/110 mg b.i.d. or 60 mg once daily (od) (to be reduced to 30 mg or in the presence of coded reduction factors) after an initial period of 5 days of heparin treatment.93
Elderly
In the four randomized clinical trials conducted with DOACs in AF patients undergoing PCI,6–9 the mean age of the population was ∼70 years. In the PIONEER AF-PCI6 and RE-DUAL PCI7 studies, the elderly population, i.e. aged ≥75 years, was underrepresented, representing about one-third of the global population. In a subanalysis of the RE-DUAL PCI study,94 in which patients were stratified by age < vs. ≥75 years, a significant safety interaction with DAT using 110 and 150 mg dabigatran was found in patients <75 years. A significant interaction was also discovered regarding the efficacy of DAT with 110 mg dabigatran, but not 150 mg, which was associated with a tendency to an increase in death/thromboembolic events in the ≥75 years population.94
Based on the above, the intensity of DAT, and therefore the choice of the 110 mg dabigatran dosage compared to that of 150 mg, can be adapted according to the individual bleeding risk,95 of which the ≥75 years of age is an important determinant.66 Age alone is not a criterion for dose selection with factor-Xa inhibitor DOACs,95 which should therefore be administered according to the codified criteria. To date, however, the containment of bleeding risk in AF patients undergoing PCI, including the elderly, must essentially be conducted by reducing the duration of the combined therapies, TAT and DAT, as well as on the use of clopidogrel compared to prasugrel and ticagrelor, and not on the DOAC dosage, except for dabigatran.
Long-term antithrombotic therapy
Among the first definitions of the best long-term antithrombotic strategies in patients with OAT indication and undergoing PCI is that of the ESC Guidelines on DAPT.96 These guidelines recommended discontinuation of antiplatelet therapy after 12 months and continuation with OAT (IIaB) alone on the basis of the Danish registry,97 which demonstrated that the long-term addition of an antiplatelet agent to VKAs, in patients with CCS, was not associated with a reduction in coronary or atherothrombotic events, but with a significant increase of the bleeding risk.
In the same guidelines,96 a possible long-term OAT-antiplatelet combination was also suggested in two cases: (i) patients at very high risk of recurrent stent-related coronary events (e.g. >3 implanted stents, diffuse multivessel disease, bifurcations treated with two stents, previous episodes of stent thrombosis despite adequate antiplatelet therapy, stents length >60 mm, stenting of the last remaining vessel); (iii) Patients with mechanical valve prosthesis and clinically evident coronary atherosclerosis.
Subsequently, in the North American Consensus Document,50 the indication for OAT alone after 12 months was confirmed, and in patients with high haemorrhagic risk and low ischaemic/thrombotic risk, the administration of OAT alone was anticipated to 6 months from the index event. This document also opened the possibility, in selected patients with high ischaemic/thrombotic risk and low haemorrhagic risk, of a DAT beyond 12 months.
Similar recommendations were contained in the 2020 ESC CCS Guidelines,98 which suggested to prolong DAT beyond one year in patients without high bleeding risk and with a history of myocardial infarction or high risk characteristics of recurrent ischaemic events (multivessel coronary artery disease associated with >1 of the following characteristics: diabetes, recurrent heart attack, peripheral arterial disease, chronic renal failure with eGFR 15–59 mL/min/1.73 m2) (indication IIb B).
In the 2020 ESC Guidelines on ACS without persistent ST-segment elevation and the 2021 EHRA practical Guidelines.10,78 the authors recommend, as a strategy defined as ‘by default’, to interrupt the antiplatelet at 12 months, continuing with the OAT alone. In the subgroup of patients classified as high bleeding risk according to the ARC, the transition to OAT alone is anticipated at 6 months. The continuation of the DAT beyond these terms is hypothesized in the cases reported by the 2019 ESC Guidelines on the CCS. In the same year, the ACC published a consensus document on anticoagulant and antiplatelet therapy in AF or VTE patients undergoing PCI,51 suggesting the continuation of the OAT alone after 6–12 months in stable patients or after 12 months after ACS. The possible prolongation of DAT beyond that limit was suggested only in patients at high thrombotic risk (previous heart attack, complex lesions, presence of cardiovascular risk factors, or extensive coronary artery disease). There are both registry data and randomized clinical trials that support the benefit of using the single OAT in this setting. The combined analysis of the PREFER-AF (European Prevention of thromboembolic events-European Registry in Atrial Fibrillation) and PREFER-AF PROLONGATION99 registries examined 1058 patients with a history of myocardial infarction or stenting >1 year, treated with OAT for AF, comparing the group with the OAT alone and the group with DAT. The net primary composite endpoint (major haemorrhages + ACS) was significantly higher in the second group: 7.9% vs. 4.2%/year (P = 0.048), with the largest difference relative to major haemorrhages [odds ratio (OR) 2.28, 95% CI 1.00–5.19].
The randomized AFIRE (Atrial Fibrillation and Ischaemic Events With Rivaroxaban in Patients With Stable Coronary Artery Disease) trial,100 compared rivaroxaban monotherapy with DAT (rivaroxaban + aspirin) in ∼2200 patients with AF and CCS, demonstrating a non-inferiority with regard to the composite endpoint of efficacy (stroke, pulmonary embolism, myocardial infarction, revascularization for unstable angina, and total mortality) (OR 0.72, 95% CI 0.55–0.95), compared to a superiority of rivaroxaban alone in terms of reduction of major haemorrhages (OR 0.59, 95% CI 0.39–0.89). Recent sub-analyses of AFIRE have demonstrated the benefit of continuing rivaroxaban monotherapy also in patients with a history of heart attack, stroke, or peripheral arterial disease101 and in patients with a history of PCI and stent implantation.102 Inconclusive data were obtained in the OAC-ALONE study,103 a study interrupted early due to a reduced patient enrolment, which failed to demonstrate, probably due to reduced statistical power, the non-inferiority of OAT alone compared to DAT after 1 year from PCI in about 700 patients with AF.
In conclusion, the data of the scientific literature are in favour of the use of OAT alone after 12 months of DAT. However, it should be noted that these data are relative to patients with CCS, who have a lower activation of coagulation and platelet aggregation, compared to patients with ACS, even 12 months after the acute event. Consequently, a careful use, may be considered in selected patients at low bleeding risk, of a single antiplatelet therapy in combination with DOACs, even beyond the year after the index event.
As for the type of antiplatelet for the possible extension beyond the year, any oral antiplatelets to be chosen in combination with OAT in ACS are clopidogrel, ticagrelor, or prasugrel. However, prolongation beyond 1 year of ticagrelor or prasugrel is not suggested, as OAT was an exclusion criterion for the PEGASUS104 and DAPT105 trials that evaluated the efficacy and safety of DAPT in other clinical settings. In conclusion, in patients with ACS, as well as for patients with CCS, the antiplatelet drugs suggested in the long term in combination with OAT are clopidogrel as first hypothesis104 or aspirin, if clopidogrel is not tolerated or if a reduced response to the drug is hypothesized.
Disclaimer
This Position Paper was originally published in the Italian language as ‘Position paper ANMCO: Trattamento antitrombotico nei pazienti con fibrillazione atriale e sindromi coronariche acute o croniche sottoposti ad angioplastica coronarica con impianto di stent’, in the official journal of the Italian Federation of Cardiology (IFC) ‘Giornale Italiano di Cardiologia’, published by Il Pensiero Scientifico Editore. This paper was translated by a representative of the Italian Association of Hospital Cardiologists (ANMCO) and reprinted with permission of IFC and Il Pensiero Scientifico Editore.
Funding
None.
Conflict of interest: L.D.L. declares to have received compensation for presentations at conferences and/or advisory boards from Aspen, AstraZeneca, Bayer, Boehringer Ingelheim, Chiesi, Daiichi Sankyo, Pfizer/Bristol-Myers Squibb, and Sanofi; A.R. declares to have received compensation for presentations at conferences and/or advisory boards from da Astra Zeneca, Bayer, Boehringer Ingelheim, Daichi Sankyo, and Pfizer/Bristol/Myers Squibb; M.L. declares to have received compensation for presentations at conferences and/or advisory boards from da Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Pfizer, and Sanofi Aventis; S.L. declares to have received compensation for presentations at conferences and/or advisory boards from da Bristol Myers Squibb, Daiichi Sankyo, Pfizer, Sanofi Aventis, AstraZeneca, Chiesi, The Medicine Company, and Bayer; M.T. declares to have received compensation for presentations at conferences and/or advisory boards from Aspen Ukraine. The other authors declare no conflicts of interest.
References
The ACTIVE Writing Group on behalf of the ACTIVE Investigators.
Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx P, Valgimigli M.
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. ESC Scientific Document Group.
De Luca L, Mistrulli R, Veneziano FA, Grigioni F, Volpe M, Musumeci F, Gabrielli D.
Singer DE, Chang Y, Borowsky LH, Fang MC, Pomernacki NK, Udaltsova N, Reynolds K, Go AS.
Zwart B, Bor WL, de Veer AJWM, Mahmoodi BK, Kelder JC, Lip GYH, Bhatt DL, Cannon CP, Ten Berg JM.
Ten Berg JM, Steg PG, Bhatt DL, Hohnloser SH, de Veer A, Nordaby M, Miede C, Kimura T, Lip GYH, Oldgren J, Cannon CP; RE-DUAL PCI Steering Committee and Investigators.
Matsuzawa Y, Kimura K, Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, Miyauchi K, Hagiwara N, Hirayama A, Matsui K, Ogawa H; AFIRE Investigators.
Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS; PEGASUS-TIMI 54 Steering Committee and Investigators.



