-
PDF
- Split View
-
Views
-
Cite
Cite
Giuseppe Patti, Chiara Ghiglieno, Prevention of ischaemic events in subjects with polydistrict vascular disease, European Heart Journal Supplements, Volume 23, Issue Supplement_E, October 2021, Pages E103–E108, https://doi.org/10.1093/eurheartj/suab102
Close - Share Icon Share
Abstract
The incidence of new cardiovascular events in patients with chronic coronary syndrome remains high, particularly in the presence of concomitant high thrombotic risk factors (diabetes mellitus, renal failure, multivessel coronary artery disease, multiple district atherosclerosis, recurrent events, heart failure). The risk of such recurrent events can be reduced by implementing various strategies, which include careful individual stratification of ischaemic and haemorrhagic risk and the choice of the most appropriate antithrombotic therapy for the individual patient, also by combining aspirin with a second antiplatelet agent/a low-dose anticoagulant, in order to achieve the maximum net clinical benefit.
Evolution of antithrombotic therapy in chronic coronary syndrome
After 50 years of research on antithrombotic strategies in patients with chronic coronary syndrome (CCS), the following fundamental conclusions can be drawn:1–3
A single antithrombotic agent (antiplatelet or anticoagulant) vs. absence of antithrombotic therapy leads to a clear reduction in the rate of myocardial infarction (AMI) and death, against an acceptable increase in the risk of bleeding.
Clopidogrel compared to aspirin (ASA) is more effective in the prevention of ischaemic events.
The risk of cardiovascular events in the follow-up of patients on single antiplatelet therapy (SAPT) remains high [up to 20% at 3 years from an acute coronary syndrome (ACS)].4,5
Dual antiplatelet therapy (DAPT) or the addition of a low-dose anticoagulant to the ASA are associated with a lower risk of ischaemic events than SAPT, at the cost of a higher incidence of major bleeding (including intracranial haemorrhage).
European Society of Cardiology guidelines on chronic coronary syndrome
The latest European Society of Cardiology (ESC) 2019 Guidelines on CCS recommend adding a second antithrombotic agent to ASA for secondary prevention in patients with ‘high’ (Class IIa-A) or ‘moderate’ (IIb-A) risk of ischaemic events, in the absence of a high risk of bleeding.6 Patients with multivessel coronary artery disease (CAD) and at least one additional risk factor are defined as ‘high risk’ including diabetes, recurrent AMI, renal failure (CRF), or peripheral vascular disease (PAD). On the other hand, patients with at least one criterion between multivessel CAD, diabetes, recurrent AMI, PAD, CRF, or heart failure are considered to be at ‘moderate’ risk.
These recommendations are the result of two fundamental randomized trials that have revolutionized the approach to antithrombotic therapy in CCS. The PEGASUS1 study, primarily conducted in patients with previous AMI, evaluated the benefit of Ticagrelor 60 mg bid vs. placebo in combination with ASA, highlighting a significant reduction in major adverse cardiovascular events (MACE), with a greater benefit in the subgroup with AMI in the 2 years prior to randomization. The latest guidelines, therefore, recommend the use of prolonged DAPT in patients with previous AMI who have tolerated 12 months of DAPT.
The COMPASS3 study, on the other hand, included patients with PAD or CAD [including multivessel CAD, previous MI, coronary angioplasty (percutaneous coronary intervention, PCI), or coronary artery bypass grafting]. In addition, 20% of patients with CAD also had PAD. The trial showed a favourable risk–benefit ratio with the use of rivaroxaban at the vascular dose (2.5 mg bid) associated with ASA vs. ASA in monotherapy, to the point of being prematurely discontinued due to excess efficacy. In COMPASS patients, rivaroxaban was associated with a reduction in total and cardiovascular mortality. Although the predetermined significance thresholds for cardiovascular and total mortality have not been reached, it is true that the study was interrupted early, after an average follow-up of 23 months (about a year earlier than expected), precisely because of the clear superiority of the rivaroxaban arm. Furthermore, in view of the early divergence of ischaemic event curves, it is plausible that the real benefit of dual antithrombotic therapy with rivaroxaban + ASA on mortality was underestimated by the early termination of the trial.
Current ESC guidelines, in evaluating the indication for long-term antithrombotic regimens in combination with ASA in patients with CCS, recommend all antithrombotic drugs to be combined with aspirin (clopidogrel, prasugrel, ticagrelor, rivaroxaban) on the same level, without distinctions based on the type of molecule.6 The definition of ‘high ischaemic risk’ adopted by the current guidelines is very similar to the COMPASS inclusion criteria. Furthermore, the reduction in mortality highlighted in COMPASS, as well as the strength of the evidence from the various randomized studies that have evaluated different dual antithrombotic approaches in patients with CCS, should be considered when choosing the antithrombotic regimen to add to the ASA, especially in patients with multidistrict atherosclerotic disease (Figure 1). Finally, the combination of rivaroxaban at a vascular dose + ASA may represent the only option for patients with CAD without a previous history of AMI.
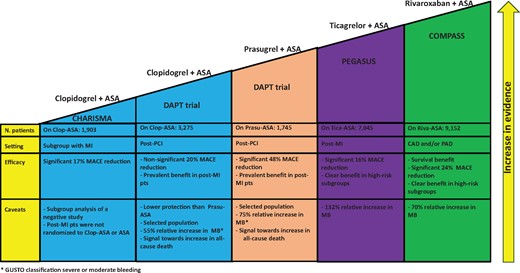
Current evidence from randomized studies on different antithrombotic approaches on top of aspirin for secondary cardiovascular prevention in patients with chronic coronary syndrome. ASA, aspirin; MACE, major adverse cardiovascular events; MB, major bleeding; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention.
The term ‘CCS’, however, encompasses an extreme variety of patients with heterogeneous levels of residual risk, from stable subjects with a remote history of CAD to patients in DAPT for an ACS 1 year earlier. This therefore entails difficulties in assessing the ‘net’ benefit of the various antithrombotic strategies in the individual patient, as the risk/benefit of each of these can vary considerably according to the clinical situation.
Personalization of the antithrombotic strategy based on the individual ischaemic risk
Following an acute cardiovascular event certain subgroups of patients have an increased risk of cardiovascular recurrence. In the context of secondary prevention, most trials agree that the extent of atherosclerotic disease (multivessel CAD or CAD with concomitant PAD) with at least one additional risk factor including medically treated diabetes mellitus, recurrent AMI, or CRF (estimated glomerular filtration rate <60 mL/min/1.73 m2) is associated with an increased risk of relapse.7,8 A more aggressive antithrombotic strategy with prolonged DAPT or dual pathway inhibition (antiplatelet therapy associated with anticoagulant at vascular dose) is associated with a greater benefit in terms of absolute reduction of ischaemic events in patients with high baseline risk. It is therefore crucial to identify the subgroups of patients at higher risk, in which specific antithrombotic therapeutic strategies are associated with a greater reduction in the risk of ischaemic complications compared to an acceptable bleeding risk.
In the PEGASUS study, the greatest absolute reduction in MACE with the use of ASA plus ticagrelor vs. ASA alone was observed in patients with prior AMI and concomitant PAD, i.e. ∼5% of the population, where the absolute reduction was 4.1% vs. 1.3% in the total trial population, and in patients with CRF (absolute reduction of 2.7%), while in the subgroup of subjects with multivessel CAD or diabetes the absolute reduction in MACE differed slightly (reduction of 1.5% in both subgroups).7,8
In the COMPASS study, renal dysfunction and diabetes were significant predictors of MACE. A rivaroxaban + ASA vs. ASA monotherapy strategy was associated with a relative benefit in terms of prevention of ischaemic events, regardless of the presence of diabetes and with any level of renal filtrate.9 The extent of atherosclerosis—both coronary and peripheral—correlates with the degree of atherosclerotic disease: the greater the number of affected vessels, the greater the risk of plaque rupture, with consequent acute thrombotic ischaemic event, particularly in the presence of diabetes and renal dysfunction. Both of these conditions, in fact, are associated with endothelial dysfunction, increased oxidative stress, and a pro-inflammatory state, responsible for the appearance of a pro-thrombotic diathesis and possible triggering of acute thrombotic events, especially in the presence of widespread atherosclerotic lesions.
Patients with PAD are therefore at an increased risk of cardiovascular events, including IMA. In fact, observational studies have shown that the risk of AMI and cardiovascular death in patients with PAD, without a history of CAD, is not very different from that of patients with documented CAD.10 In addition, subjects with PAD are at greater risk of major adverse limbs events (MALE), in particular acute limb ischaemia requiring amputation. A linear increase in the MACE rate was also highlighted with the number of vascular sites affected by atherosclerosis. In these patients it is therefore essential to reduce both MACE and MALE. In the COMPASS study, the greatest absolute reduction in MACE in the rivaroxaban + ASA arm was observed in patients with concomitant CAD and PAD (−2.7%). In patients with polydistrict vascular disease, the dual pathway inhibition strategy showed a 6% decrease in the absolute risk of the composite endpoint of MACE, acute limb ischaemia, and total amputations, and a 5.9% decrease in the absolute risk of net clinical endpoint including cardiovascular death, stroke, MI, acute limb ischaemia, vascular amputation, fatal, or symptomatic major organ bleeding.3,9,11
Finally, it is appropriate to underline how many conditions associated with an increased thrombotic risk can also predispose to bleeding (e.g. old age, diabetes, or CRF). To evaluate the effects of antithrombotic strategies, therefore, it appears crucial to balance protection from ischaemic events with the concomitant increase in bleeding risk, trying to highlight the ‘net clinical benefit’ in the individual patient. Assuming that the ‘net clinical benefit’ derives from the balance between efficacy (i.e. the amount of reduction in ischaemic events, with a high baseline risk corresponding to a greater expected efficacy) and safety (understood as the extent of the risk of bleeding, with a minimum risk of baseline bleeding corresponding to greater expected safety), it is essential to identify those subgroups of patients with high ischaemic risk and/or low haemorrhagic risk.
Risk stratification scores
After 12 months from an acute coronary event, several randomized trials have shown that prolongation of the DAPT is not beneficial in all patients; the patients who seem to benefit most from prolonged DAPT regimens, in fact, are those who have a high ischaemic risk in the absence of an increased risk of bleeding.
To facilitate risk stratification and maximize the net clinical benefit of antithrombotic strategies, some scores were therefore developed specifically for patients who completed the 1-year post-ACS follow-up (Table 1). The TRS2°P (TIMI Risk Score for Secondary Prevention), developed from the TRA2° P-TIMI trial and based on nine clinical parameters, showed a moderate stratification power of patients with previous AMI regarding the composite endpoint of recurrent AMI, ischaemic stroke, and cardiovascular death, identifying patients at high ischaemic risk with a score >3 points.12 The DAPT score, derived from the DAPT trial, represents another useful score for the stratification of the subgroups of patients able to benefit most from regimens of prolonged DAPT 1 year after the acute event (those with ≥2 points), in which the ‘number needed to treat’ was 34 for the ischaemic endpoint and the ‘number needed to harm’ for bleeding was 272.13
| Score . | Clinical setting . | Variables included . | Events included in outcome . | Timing of outcome . |
|---|---|---|---|---|
| Ischemic risk | ||||
| TRS2°P | Previous MI in the last 12 months |
| Recurrent MI, ischemic stroke, cardiovascular death | 3 years |
| Bleeding risk | ||||
| PRECISE-DAPT | At the time of coronary stenting |
| TIMI major bleeding; any TIMI bleeding | 1 year |
| Ischemic and bleeding risk | ||||
| DAPT score | At least 1 year from STEMI/NSTE-ACS |
| MI or stent thrombosis; GUSTO moderate or severe bleeding | 30 months after the index event |
| Score . | Clinical setting . | Variables included . | Events included in outcome . | Timing of outcome . |
|---|---|---|---|---|
| Ischemic risk | ||||
| TRS2°P | Previous MI in the last 12 months |
| Recurrent MI, ischemic stroke, cardiovascular death | 3 years |
| Bleeding risk | ||||
| PRECISE-DAPT | At the time of coronary stenting |
| TIMI major bleeding; any TIMI bleeding | 1 year |
| Ischemic and bleeding risk | ||||
| DAPT score | At least 1 year from STEMI/NSTE-ACS |
| MI or stent thrombosis; GUSTO moderate or severe bleeding | 30 months after the index event |
CrCl, creatinine clearance; DAPT, dual antiplatelet therapy; eGFR, estimated glomerular filtration rate; GUSTO, Global Strategies for Opening Occluded Coronary Arteries; MI, myocardial infarction, NSTE-ACS, non ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; PRECISE-DAPT, PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual AntiPlatelet Therapy; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction; TRS2°P, TIMI Risk Score for Secondary Prevention.
| Score . | Clinical setting . | Variables included . | Events included in outcome . | Timing of outcome . |
|---|---|---|---|---|
| Ischemic risk | ||||
| TRS2°P | Previous MI in the last 12 months |
| Recurrent MI, ischemic stroke, cardiovascular death | 3 years |
| Bleeding risk | ||||
| PRECISE-DAPT | At the time of coronary stenting |
| TIMI major bleeding; any TIMI bleeding | 1 year |
| Ischemic and bleeding risk | ||||
| DAPT score | At least 1 year from STEMI/NSTE-ACS |
| MI or stent thrombosis; GUSTO moderate or severe bleeding | 30 months after the index event |
| Score . | Clinical setting . | Variables included . | Events included in outcome . | Timing of outcome . |
|---|---|---|---|---|
| Ischemic risk | ||||
| TRS2°P | Previous MI in the last 12 months |
| Recurrent MI, ischemic stroke, cardiovascular death | 3 years |
| Bleeding risk | ||||
| PRECISE-DAPT | At the time of coronary stenting |
| TIMI major bleeding; any TIMI bleeding | 1 year |
| Ischemic and bleeding risk | ||||
| DAPT score | At least 1 year from STEMI/NSTE-ACS |
| MI or stent thrombosis; GUSTO moderate or severe bleeding | 30 months after the index event |
CrCl, creatinine clearance; DAPT, dual antiplatelet therapy; eGFR, estimated glomerular filtration rate; GUSTO, Global Strategies for Opening Occluded Coronary Arteries; MI, myocardial infarction, NSTE-ACS, non ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; PRECISE-DAPT, PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual AntiPlatelet Therapy; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction; TRS2°P, TIMI Risk Score for Secondary Prevention.
The ESC guidelines on CCS define as ‘high risk of bleeding’ patients with at least one criterion between previous intracranial haemorrhage/ischaemic stroke/intracranial pathology, recent gastrointestinal bleeding/anaemia due to possible gastrointestinal bleeding/presence of high-risk disease for gastrointestinal bleeding/hepatic insufficiency/haemorrhagic diathesis/coagulopathy, advanced age or frailty, CRF with glomerular filtration rate <15 mL/min/1.73 m2 (or on dialysis therapy).6
The PRECISE-DAPT score was recently developed to identify patients with high bleeding risk and who could therefore benefit from a shorter DAPT regimen following PCI (3–6 vs. 12–24 months).14 In a recent analysis, patients in the PRECISE-DAPT trial were divided into high- or low-risk bleeding patients (PRECISE-DAPT ≥25 and <25, respectively) and high or low ischaemic risk patients based on the complexity of coronary revascularization; it was thus found that in the subgroup with high bleeding risk, regardless of ischaemic risk, prolonged DAPT was associated with an increase in bleeding complications, without a benefit on MACE or 2-year mortality. Haemorrhagic risk stratification influenced prognosis more than ischaemic risk.
Finally, it is important to consider that the bleeding risk can be dynamic: the factors predisposing to bleeding, in fact, can vary and/or weigh differently in the various patients and be modifiable (and also vary according to the type of antithrombotic regimen chosen).
Balance between antithrombotic ischaemic protection and haemorrhagic risk in chronic coronary syndrome
Definition of bleeding events in trials
A variety of different definitions of ‘bleeding’ have been used in the various trials of antithrombotic therapies. This lack of standardization therefore complicates the comparison between safety and efficacy endpoints in the different studies. In a recent analysis of patients enrolled in ENGAGE AF-TIMI 48, for example, an up to four times higher rate of ‘serious’ bleeding was observed with reference to the different bleeding classifications used.15
The definition of TIMI major bleeding, for example, includes lethal bleeding, intracranial haemorrhage, drop in haemoglobin (Hb) ≥5 g/dL or haematocrit ≥15%. On the contrary, according to the ISTH scale, ‘major bleeding’ is defined by fatal bleeding, symptomatic bleeding in a critical area or which determines a reduction in Hb ≥2 g/dL or the need for transfusion of at least two units of red blood cells. Thus it happens that a major bleeding according to ISTH may have less relevance than one based on the TIMI definition. The difficulty in comparing the events between trials adopting different definitions of bleeding complications is therefore evident. In PEGASUS, for example, bleeding was quantified on the basis of the TIMI definition, while in COMPASS a modified ISTH scale was used. With the aim of solving this problem, the Academic Research Consortium recently proposed a standardized classification for all bleeding events (BARC scale).
Quantify the severity of bleeding events
Bleeding complications related to antithrombotic therapies are associated with an adverse prognosis, including an increased risk of AMI, stroke, intrastent thrombosis, and death. The mechanisms underlying the increase in morbidity/mortality associated with bleeding events are only partly attributable to the severity of the bleeding, which directly impacts the prognosis (in particular in the case of intracranial Haemorrhage). Other factors, however, contribute to bleeding-related mortality and morbidity, such as the increased incidence of coronary events following discontinuation of antithrombotic therapies and/or hypotension, adverse outcomes of hyper-adrenergic state, secondary pro-inflammatory, and immunological effects to transfusions.
In the context of ‘post-ACS’ it emerged that the mortality related to bleeding is lower than that related to an MI for non-serious bleeding (BARC 2 and 3a), but can be up to four times higher in the case of severe bleeding (BARC 3c).16 It has also been shown that in the context of CCS the incidence of bleeding complications can vary over time. In fact, in the rivaroxaban + ASA arm of the COMPASS study, while the reduction in MACE (compared to monotherapy with ASA) remained constant throughout the study, the relative increase in ‘severe’ bleeding was prevalent in the first year of follow-up and almost entirely confined to the first 2 years.3 A similar result, although less marked, was also observed in PEGASUS, with the combination of ticagrelor 60 mg bid + ASA vs. monotherapy with ASA.1
Net clinical benefit of antithrombotic strategies in secondary prevention
The concept of ‘net clinical benefit’ appears to be the most relevant in the context of CCS, since in this ‘clinical setting’ the goal of treatment is chronic risk reduction, and not overcoming an acute ischaemic event. A crucial point is represented by the evaluation of the actual clinical weight of ischaemic events, compared to haemorrhagic ones, included in the composite endpoints, managing to identify those events with the greatest impact on prognosis and mortality. In the COMPASS3 trial, the incidence of the net clinical endpoint (cardiovascular death, stroke, MI, fatal, or symptomatic critical site bleeding) was lower in the rivaroxaban + ASA vs. ASA monotherapy arm (HR 0.80, P < 0.001), thus likely causing the observed reduction in mortality. Similarly, an analysis of PEGASUS showed a significant relative reduction of 14% in the net composite endpoint of cardiovascular death, stroke, MI, fatal bleeding, or intracranial haemorrhage in the ticagrelor + ASA vs. ASA monotherapy arm.1
Figure 2 summarizes a practical approach to the factors to be considered for the choice of antithrombotic therapy in the patient with CCS.
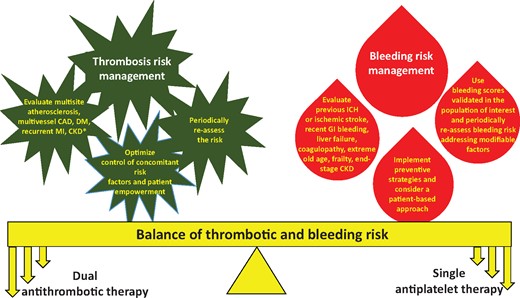
Practical approach to the factors to be evaluated for the choice of antithrombotic treatment in the patient with chronic coronary syndrome. CAD, coronary artery disease; CKD, chronic kidney disease; DM, diabetes mellitus; GI, gastro-intestinal; ICH, intracranial haemorrhage; MI, myocardial infarction.
Conclusions
The incidence of recurrent cardiovascular events in patients with CCS remains high, especially in the presence of a high thrombotic risk. The risk of recurrent events can be reduced by implementing various strategies, such as careful individual stratification of ischaemic and haemorrhagic risk, the choice of the most appropriate antithrombotic therapy for the individual patient, in order to achieve the maximum net clinical benefit and the execution of regular follow-ups, with periodic reassessments of the risk profile, in consideration of its dynamism.
Conflict of interest: none declared.



