-
PDF
- Split View
-
Views
-
Cite
Cite
Michael Böhm, Felix Mahfoud, Christian Werner, Koon Teo, Magnus Baumhäkel, Cardiovascular protection: a breakthrough for high-risk patients, European Heart Journal Supplements, Volume 11, Issue suppl_F, December 2009, Pages F19–F26, https://doi.org/10.1093/eurheartj/sup024
Close - Share Icon Share
Abstract
Blockade of the renin–angiotensin system (RAS) with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (ARBs) has been shown to reduce cardiovascular mortality and morbidity in various patient populations. The recent ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET) and Telmisartan Randomized AssessmeNt Study in aCE iNtolerant subjects with cardiovascular Disease (TRANSCEND) studies with the ARB telmisartan, the largest outcome trial programme with an ARB, have extended this evidence base in the broadest cross section of cardiovascular high-risk patients, and provided important new insights into the benefits of RAS blockade. ONTARGET and TRANSCEND recruited patients who were at high risk of vascular events. These patients, who did not have established heart failure and had well controlled blood pressure, had not previously been studied in clinical trials with ARBs. Telmisartan provided cardiovascular protection similar to ramipril but was better tolerated. These studies add to the growing evidence that the effects of RAS inhibitors are not solely dependent on blood pressure reduction. Angiotensin II exerts diverse pro-atherosclerotic effects, and hence blockade of the RAS may directly inhibit the development and progression of atherosclerosis. Finally, together with other smaller outcomes studies, ONTARGET and TRANSCEND provide useful insights into the relative importance of hypertension and other risk factors at different stages of the ‘cardiovascular continuum’. The evidence suggests that high blood pressure has a major impact on cardiovascular risk in the early stages of the continuum, whereas other risk factors like RAS activation become progressively important in later stages.
Introduction
Activation of the renin–angiotensin system (RAS) has multiple deleterious effects on the heart and blood vessels, including vasoconstriction, endothelial dysfunction leading to subsequent hypertension as well as cardiac fibrosis and myocardial remodelling.1 As a result, RAS activation is a major risk factor for cardiovascular mortality and morbidity, and modulators of this system now hold a central position in the treatment of patients at high cardiovascular risk.2–5 Randomized controlled trials have shown that inhibition of the RAS with angiotensin-converting enzyme (ACE) inhibitors prevents cardiovascular events such as cardiac death, myocardial infarction, or stroke in patients with left ventricular dysfunction or heart failure,6–8 patients with a history of vascular disease,9,10 and high-risk diabetic patients.11 Similarly, studies with angiotensin receptor blockers (ARBs) have shown that these agents reduce the risk of death or hospitalization for heart failure in patients with left ventricular dysfunction or heart failure,12–14 and are significantly more effective than beta-blockers in reducing vascular events among high-risk patients with hypertension and left ventricular hypertrophy (LVH).15 Data on the combination of ARBs and ACE-inhibitors in secondary prevention were lacking but controversially discussed.16
This comprehensive evidence base has recently been expanded by the ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET)17 and Telmisartan Randomized AssessmeNt Study in aCE iNtolerant subjects with cardiovascular Disease (TRANSCEND)18 studies, which have shown that the ARB telmisartan is as effective as the ACE inhibitor ramipril in reducing cardiovascular events in high-risk patients without heart failure, and is significantly more effective than placebo in preventing the composite of cardiovascular death, myocardial infarction and stroke in patients who are unable to tolerate ACE inhibitors. The implications of these studies for clinical practice are reviewed in this article.
Proof of concept: direct vascular protective effects of renin–angiotensin system inhibition
Atherosclerosis is a step-wise process, that begins with endothelial dysfunction and progresses via monocyte activation and lipid deposition to fatty streak formation and plaque development, and ultimately to the rupture of an unstable plaque and subsequent vascular events such as myocardial infarction or stroke. Angiotensin II, acting via AT1-receptors, plays an important role in each stage of the atherogenic process. Thus, angiotensin II promotes endothelial dysfunction by increasing oxidative stress through free radical formation,19 thereby decreasing nitric oxide production and hence reducing endothelial integrity. It also increases the adhesion of inflammatory cells to vascular endothelium by enhancing the expression of adhesion molecules such as intercellular adhesion molecule 1 and activating monocytes.20–23 In addition, angiotensin II induces the expression of inflammatory mediators such as interleukin-6,24 promotes fatty streak formation by increasing the formation and uptake of oxidized low density lipoprotein cholesterol,25 stimulates smooth muscle cell proliferation,19 and induces vascular smooth muscle cell apoptosis26 and matrix metalloprotease expression,27 both of which may contribute to plaque rupture.
There is substantial evidence that inhibition of the RAS has beneficial effects on atherosclerosis. ACE has been shown to be present in inflammatory cells and vascular endothelial cells within atherosclerotic plaques,28 and in animal studies RAS inhibitors prevent intimal proliferation following vascular injury29,30 and the development of experimental atherosclerosis.31–33 Direct evidence that angiotensin II acts via AT1-receptors to promote atherogenesis comes from studies in knock-out mice lacking the AT1 and apolipoprotein E (apoE) receptors.34 In mice lacking the apoE receptor, administration of a high-cholesterol diet resulted in the development of endothelial dysfunction and atherosclerotic lesions. Importantly, these effects occurred in the absence of changes in blood pressure. In contrast, in mice that also lacked AT1-receptors, the administration of a high-cholesterol diet did not result in the development of atherosclerosis, again, at similar blood pressure levels.
Such findings suggest that inhibitors of the RAS may exert beneficial effects on cardiovascular risk through mechanisms that are at least partly independent of blood pressure. Clinical evidence in favour of this notion comes from studies such as SOLVD (Studies Of Left Ventricular Dysfunction)7,35 and SAVE (Survival And Ventricular Enlargement),36 which showed that, in addition to decreasing mortality and morbidity associated with heart failure, ACE inhibitors reduced the incidence of vascular events in heart failure patients. Subsequently, the Heart Outcomes Prevention Evaluation (HOPE) Study,9 and the ONTARGET17 and TRANSCEND18 studies have shown that RAS blockade reduces the risk of cardiovascular events in patients with vascular disease irrespective of baseline blood pressure and subsequent blood pressure lowering. These trials can therefore be considered ‘proof of concept’ studies, demonstrating that RAS blockade can reduce cardiovascular events independently of changes in blood pressure.
Filling the evidence gap in high-risk patients
Cardiovascular disease can be regarded as a continuum that begins with risk factors such as hypertension, smoking, lipid disorders, diabetes, or obesity in conjunction with inadequate physical activity and progresses to endothelial dysfunction, atherosclerosis, and coronary heart disease, leading ultimately to LVH, myocardial infarction, and progressive left ventricular impairment (Figure 1).37 The effects of ARB therapy during the early stages of the continuum were studied in the LIFE (Losartan Intervention For Endpoint reduction in hypertension) study,15 which compared losartan and atenolol in 9193 patients with essential hypertension and LVH. The incidence of the primary endpoint, a composite of death, myocardial infarction, or stroke, was significantly lower in losartan-treated patients than in those receiving atenolol [relative risk (RR) 0.87, 95% confidence interval (CI) 0.77–0.98, P = 0.021], despite comparable reductions in blood pressure in the two groups. The incidence of stroke was also significantly lower in the losartan group (RR 0.75, 95% CI 0.63–0.89, P = 0.001), and new-onset diabetes was less common with losartan.
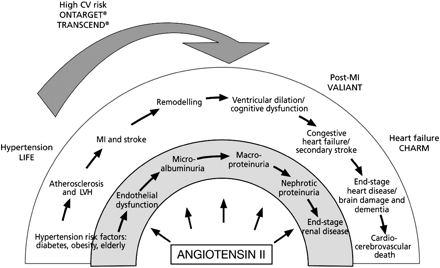
The ‘cardiovascular continuum’ of Dzau and Braunwald.37 The ONTARGET and TRANSCEND studies have filled an ‘evidence gap’ by documenting the benefits of ARB therapy in high-risk patients without overt heart failure. CV, cardiovascular; MI, myocardial infarction; LVH, left ventricular hypertrophy.
Other studies have investigated the impact of ARBs in patients with left ventricular dysfunction or heart failure. The VALsartan In Acute myocardial InfarctioN Trial (VALIANT) study compared valsartan, captopril, and the two drugs in combination, in 14 703 patients with left ventricular dysfunction or heart failure who had experienced a recent (0.5–10 days) myocardial infarction.38 During a median follow-up of 24.7 months, there was no significant difference in survival between valsartan-treated and captopril-treated patients [hazard ratio (HR) 1.00, 97.5% CI 0.90–1.11, P = 0.98], and the combination of the two drugs was not associated with an improvement in survival compared with either drug alone. These findings are in contrast to those of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) study programme,12,13 in which treatment with candesartan was associated with significant reductions in heart failure mortality and morbidity, both in patients who were already receiving ACE inhibitor therapy and in patients who were intolerant to ACE inhibitors.
Although these studies have shown that ARBs are beneficial at different points along the cardiovascular continuum, they did not address an important patient population: those who have survived a vascular event such as a myocardial infarction, and are therefore at high risk of subsequent events, but do not have established heart failure. This population differs from heart failure patients in several respects. Heart failure patients tend to be older and to die from different causes, such as sudden cardiac death or left ventricular pump failure; in addition, heart failure patients are likely to be hospitalized for different reasons than patients with acute cardiovascular events, and comorbidities such as anaemia or renal dysfunction are common. Thus, it is not possible to extrapolate findings from trials in heart failure patients to patients with vascular disease but without established heart failure.
The ONTARGET study recruited patients aged 55 years or older with coronary artery disease, peripheral vascular disease, cerebrovascular disease, or high-risk diabetes with evidence of end-organ damage.39 In these patients, the incidence of the primary endpoint, a composite of cardiovascular death, myocardial infarction, stroke, or hospitalization for heart failure, was 16.5% in ramipril-treated patients and 16.7% in those receiving telmisartan (RR 1.01, 95% CI 0.94–1.09; Figure 2).16 Importantly, the protective effects of ramipril had already been demonstrated in the HOPE study9; thus, the ONTARGET study showed that telmisartan is as effective as proven ACE inhibitor therapy in this high-risk population. Similarly, in the TRANSCEND study, in which the inclusion criteria were the same as in ONTARGET except that patients were required to be intolerant to ACE inhibitors, the incidence of cardiovascular death, myocardial infarction, or stroke (i.e. the endpoint used in the HOPE study) was reduced by 13% (95% CI 0–24%, P = 0.048) in telmisartan-treated patients, compared with the placebo group, although there was a non-significant reduction in the primary endpoint that included heart failure in addition to the components of the HOPE endpoint.18 Thus, these trials show that ARB therapy is effective or at least has a trend towards reducing the risk of cardiovascular events in a group not previously included in trials with ARBs (Figure 1). Importantly, these benefits were achieved against the background of intensive treatment of other cardiovascular risk factors. In both trials, approximately 55–62% of patients were receiving statins, 57–59% were receiving a beta-blocker, and 80–81% were receiving anti-platelet therapy.17,18
ONTARGET and TRANSCEND are important because of the patient population that was studied. Extrapolated from recent data from the American Heart Association,40 high-risk patients without heart failure represent a substantial number of patients in routine clinical practice. These data suggest that in the USA alone there are ∼21 million patients at high cardiovascular risk, the majority of which might meet the inclusion criteria for ONTARGET and TRANSCEND. In contrast, there are only 7.2 million with myocardial infarction and left ventricular impairment (as studied in the VALIANT trial), and 5 million with overt heart failure (as studied in the CHARM trials). This high-risk group therefore might constitute a substantial number of patients in routine clinical practice.
Tolerability during long-term treatment: an important consideration in high-risk patients
It is important to note that patients, such as those in the ONTARGET and TRANSCEND studies, who have experienced a cardiovascular event can have a long life expectancy. There may be an interval of up to 30 years between the initial event and the development of end-stage heart failure. Hence, cardioprotective treatment may need to be maintained for many years in these patients. This raises the question of how adherence to therapy can best be maintained during such long-term treatment, which in turn focuses attention on the importance of long-term tolerability.
It is well established that adherence to treatment for chronic conditions is often poor. For example, in an analysis of prescription records from a large pharmaceutical benefits management organization in the USA, the proportion of patients who were still taking their original anti-hypertensive medication after 12 months was only 48%.41 The highest persistence rates were seen with ARBs (64%) and ACE inhibitors (58%); in contrast, hydrochlorothiazide and beta-blockers were associated with persistence rates of only 38 and 43%, respectively. Reasons for non-persistence with antihypertensive therapy were investigated in a questionnaire study involving 1603 patients who had changed their medication within the previous 6 months.42 This study showed that adverse events were the principal reason for changing medication: 30% of patients switched medications primarily because of adverse events. Medication was changed because of patient dissatisfaction in a further 20% of patients, and because of non-compliance in 17%.
Poor tolerability is clearly an important cause of non-persistence with therapy. This is particularly true for conditions, such as hypertension, that are usually asymptomatic. Studies have consistently shown that ARBs offer an outstanding, placebo-like tolerability profile,43 making these agents a suitable choice for long-term cardioprotective treatment. In the ONTARGET study, telmisartan was associated with significantly lower incidences, compared with ramipril, of cough (1.1 vs. 4.2%, P < 0.001) and angio-oedema (0.1 vs. 0.3%, P = 0.01). However, hypotensive symptoms, which were not generally associated with treatment discontinuations, were more frequent (2.6 vs. 1.7%, P < 0.001) in patients receiving the ARB, reflecting the greater reduction in blood pressure achieved with telmisartan. In the TRANSCEND study, the time to permanent discontinuation of treatment because of adverse events was longer with telmisartan than with placebo (Figure 3), which may indicate that telmisartan was better tolerated than the concomitant medications (principally beta-blockers, statins, and anti-platelet agents) used in the placebo group at increasing frequency over the study period. Indeed, the tolerability of telmisartan may actually have been under-estimated in this study because patients who had previously been found to be intolerant to ACE inhibitors (for example, because of hypotension or hyperkalaemia) were excluded.
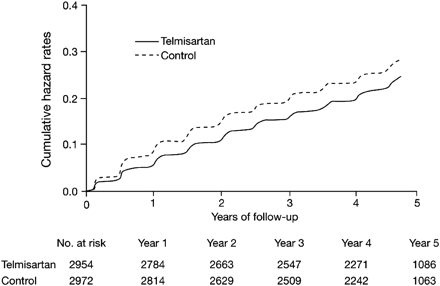
Kaplan–Meier plot showing the time to permanent discontinuation of study treatment because of adverse events in patients treated with telmisartan or placebo in addition to current best standard of care in the TRANSCEND study.
Mechanisms underlying cardiovascular events in high-risk patients: the role of hypertension
The ONTARGET and TRANSCEND studies may provide useful insights into the pathophysiological mechanisms underlying cardiovascular events in high-risk patients without heart failure, and the impact of hypertension as a risk factor in this group. It is important to note that ONTARGET and TRANSCEND were not hypertension trials.46 Approximately one-third (32.7%) of patients in ONTARGET, and one-quarter (24.1%) of those in TRANSCEND, were not hypertensive at baseline, and mean blood pressure at randomization was 134/77 and 135/78 mmHg, respectively. Thus, blood pressure, unlike in hypertension trials, was well controlled in the patients randomized in these trials. This would suggest that the benefits of telmisartan in these studies, and of ramipril in ONTARGET, were unlikely to be attributable to blood pressure reduction alone. Furthermore, in the ONTARGET study the combination of telmisartan and ramipril produced a greater reduction in blood pressure than ramipril alone, but was not associated with a decreased risk of cardiovascular events, compared with the ACE inhibitor alone. Similarly, subgroup analyses showed no significant benefit of combination treatment, compared with ramipril alone, in subgroups stratified according to systolic blood pressure (≤134, 134–150, >150 mmHg).17
Such findings might imply that the impact of blood pressure on cardiovascular risk varies at different points on the cardiovascular continuum. Importantly, epidemiological data suggest that the contribution of blood pressure (in the range of 115–190 mmHg) to mortality from ischaemic heart disease is comparable among patient subgroups stratified by age (in decades from 40 to 90 years; Figure 4).44 Furthermore, even ‘normal’ (120–129/80–84 mmHg) or ‘high normal’ (130–139/85–89 mmHg) blood pressure is associated with an increased risk of cardiovascular disease, compared with ‘optimal’ (<120/80 mmHg) blood pressure.45 Comparison of such epidemiological data with the results of outcome trials with ACE inhibitors or ARBs shows that mortality varies widely between populations: 2-year mortality in patients with hypertension, or in high-risk patients such as those in ONTARGET/TRANSCEND, is approximately 5%, whereas in patients with severe heart failure 2-year mortality exceeds 60% (Figure 5).
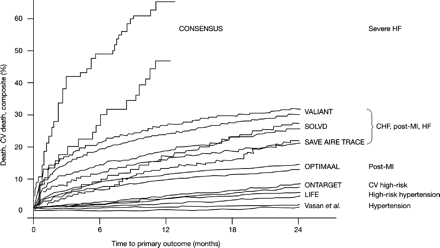
Overall and cardiovascular mortality at 2 years in patients with ‘normal’ or ‘high-normal’ blood pressure,45 and in patients enrolled in major outcome trials with ACE inhibitors or ARBs. CONSENSUS, Cooperative North Scandinavian Enalapril Survival Study3,50; AIRE, Acute Infarction Ramipril Efficacy51; TRACE, Trandolapril Cardiac Evaluation52; OPTIMAAL, Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan53; for other acronyms and references, see text.
A recent analysis of data from the ONTARGET study shows that only patients in the highest quartile of blood pressure (systolic blood pressure >154 mmHg) had a significantly increased risk of the primary endpoint (cardiovascular death, myocardial infarction, stroke, or hospitalization for heart failure), and that there were no significant differences in the risk of cardiovascular death or myocardial infarction between the quartiles.46 This suggests that the risk factors contributing to death and morbidity in high-risk patients such as those in ONTARGET and TRANSCEND may differ from those operating in heart failure patients. Further evidence for this comes from the consistent finding in heart failure registries that mortality is higher among heart failure patients admitted with a low blood pressure than in those with higher blood pressures. For example, in a prospective observational study in 206 Italian cardiology centres, in-hospital mortality was 15.3% in patients with systolic blood pressures below 119 mmHg, and decreased progressively to 2.2% among patients with systolic blood pressures of 161 mmHg or higher.47 This might suggest that high blood pressure has a major impact on cardiovascular risk in the early stages of the cardiovascular continuum, but other risk factors, such as RAS activation, dyslipidaemia, sympathetic nervous system activation, vascular oxidative stress and increased cytokine expression,48,49 become progressively more important in later stages.
Conclusions
The ONTARGET and TRANSCEND studies have provided valuable information about the benefits of telmisartan in comparison with ramipril9,17,18 in preventing cardiovascular events in high-risk patients without established heart failure, a population previously unrepresented in clinical trials with ARBs. Telmisartan was well tolerated, which is important for secondary prevention in patients who will require cardiovascular protection for many years to come. These studies have also extended our understanding of the pathophysiological role of the RAS in cardiovascular disease. It is clear that the benefits of telmisartan and ramipril in these studies are not solely related to blood pressure reduction, and that RAS inhibition results in direct protective effects on the heart and vasculature. This recognition in turn offers the potential for increased understanding of the mechanisms underlying cardiovascular risk at different stages of the cardiovascular continuum. ONTARGET and TRANSCEND add to the evidence that risk factors other than hypertension may play increasing roles in the later stages of the continuum, highlighting the importance of global cardiovascular risk assessment, and the necessity of adopting a multifactorial approach to risk management, in which RAS inhibition plays a central role.
Conflict of interest: M. Böhm was on the steering committee of ONTARGET and has received speaking honoraria from Boehringer Ingelheim and other companies. K.K.T. has received research grants, honoraria and consultation fees from Boehringer Ingelheim. F.M, C.W, and M. Baumhäkel have no conflict of interest to declare.
References
- angiotensin-converting enzyme inhibitors
- atherosclerosis
- ramipril
- hypertension
- cardiovascular diseases
- renin-angiotensin system
- heart disease risk factors
- angiotensin-converting enzyme
- angiotensin ii
- angiotensin receptor antagonists
- heart failure
- blood pressure
- physical examination
- cardiovascular system
- morbidity
- telmisartan
- evidence-based practice
- cardiovascular death
- ontarget trial





