-
PDF
- Split View
-
Views
-
Cite
Cite
Q Ma, Y Du, J.L Wang, S.J Wu, Y.X Zhao, Y.J Zhou, Long-term safety and performance of a novel conical bioresorbable vascular scaffold: insights from in-vitro and in-vivo studies, European Heart Journal, Volume 41, Issue Supplement_2, November 2020, ehaa946.2538, https://doi.org/10.1093/ehjci/ehaa946.2538
Close - Share Icon Share
Abstract
The phenomenon of size-mismatches between cylindrical stents and tapered vessels is not uncommon in current endovascular interventions which is associated with poor clinical outcomes.
The aim of the present study was to evaluate the mechanical properties of the novel conic BRS and to validate its performance with the support of optical coherence tomography (OCT), quantitative coronary angiography (QCA) and histology up to 2 years in a porcine model.
We produced the conical BRS with the four-axis 3D printing system, with a computer-controlled rotational axis (the 4th axis) in addition to the 3 axes of traditional 3D printing systems.
Mechanical properties were evaluated by recoil and radial strength, cyclic fatigability testing.
Twelve swine that received 12 conic BRS were evaluated by OCT, QCA and histology post-implantation and at 12 and 24 months.
The in vitro study showed no fractures after accelerated cycle testing over time (at 3.8×108 cycles).
The recoil rate of the scaffolds after plate compress test was 14.3±0.61%.
There was no significant peri-operative complications.
By OCT, 60±21 struts per BRS were recognizable by 2 years. Quantitative coronary angiography showed late luminal loss and percent diameter stenosis were 0.02±0.52 mm and 0.50±16.90% at 2-year follow-up.
Histopathological analysis demonstrated mild vessel injuries, inflammatory cell infiltration around struts at 1 and 2 years follow ups.
The conical BRS showed optimal performance and has the potential to improve clinical outcome.
QCA results
| . | Entire stented segment . | Proximal segment . | Middle segment . | Distal segment . |
|---|---|---|---|---|
| Post-implantation (n=12) | ||||
| Mean lumen diameter (mm) | 3.38±0.11 | 3.61±0.12 | 3.39±0.10 | 3.14±0.12 |
| Stent-vessel ratio | 1.03±0.06 | 1.03±0.08 | 1.03±0.09 | 1.02±0.02 |
| Min lumen diameter (mm) | 3.13±0.15 | 3.33±0.15 | 3.12±0.16 | 2.93±0.16 |
| 1 year follow up (n=12) | ||||
| Mean lumen diameter (mm) | 3.04±0.15 | 3.28±0.16 | 3.02±0.13 | 2.82±0.18 |
| Min lumen diameter (mm) | 2.75±0.20 | 2.91±0.20 | 2.72±0.23 | 2.63±0.18 |
| Late lumen loss (mm) | 0.37±0.36 | 0.42±0.35 | 0.38±0.39 | 0.31±0.34 |
| 2 year follow up (n=9) | ||||
| Mean lumen diameter (mm) | 3.40±0.15 | 3.61±0.19 | 3.45±0.11 | 3.16±0.16 |
| Min lumen diameter (mm) | 3.04±0.37 | 3.28±0.40 | 3.06±0.31 | 2.80±0.42 |
| Late lumen loss (mm) | 0.08±0.42 | 0.06±0.43 | 0.05±0.39 | 0.11±0.44 |
| . | Entire stented segment . | Proximal segment . | Middle segment . | Distal segment . |
|---|---|---|---|---|
| Post-implantation (n=12) | ||||
| Mean lumen diameter (mm) | 3.38±0.11 | 3.61±0.12 | 3.39±0.10 | 3.14±0.12 |
| Stent-vessel ratio | 1.03±0.06 | 1.03±0.08 | 1.03±0.09 | 1.02±0.02 |
| Min lumen diameter (mm) | 3.13±0.15 | 3.33±0.15 | 3.12±0.16 | 2.93±0.16 |
| 1 year follow up (n=12) | ||||
| Mean lumen diameter (mm) | 3.04±0.15 | 3.28±0.16 | 3.02±0.13 | 2.82±0.18 |
| Min lumen diameter (mm) | 2.75±0.20 | 2.91±0.20 | 2.72±0.23 | 2.63±0.18 |
| Late lumen loss (mm) | 0.37±0.36 | 0.42±0.35 | 0.38±0.39 | 0.31±0.34 |
| 2 year follow up (n=9) | ||||
| Mean lumen diameter (mm) | 3.40±0.15 | 3.61±0.19 | 3.45±0.11 | 3.16±0.16 |
| Min lumen diameter (mm) | 3.04±0.37 | 3.28±0.40 | 3.06±0.31 | 2.80±0.42 |
| Late lumen loss (mm) | 0.08±0.42 | 0.06±0.43 | 0.05±0.39 | 0.11±0.44 |
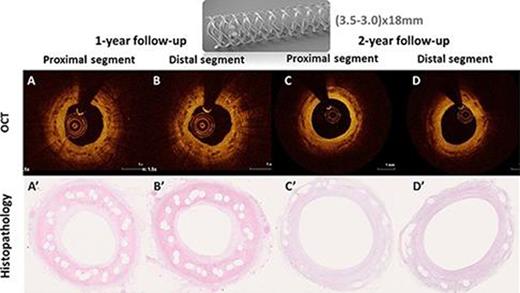
OCT and histological images
Type of funding source: None
- stents
- coronary angiography
- inflammation
- fractures
- computers
- constriction, pathologic
- follow-up
- safety
- suidae
- histology
- phenobarbital
- treatment outcome
- vascular injuries
- in vitro study
- optical coherence tomography
- histopathology tests
- endovascular procedures
- printing, three-dimensional
- mismatch
- bioresorbable vascular scaffold



