-
PDF
- Split View
-
Views
-
Cite
Cite
Andrew R Chapman, Caelan Taggart, Jasper Boeddinghaus, Nicholas L Mills, Keith A A Fox, Type 2 myocardial infarction: challenges in diagnosis and treatment, European Heart Journal, Volume 46, Issue 6, 7 February 2025, Pages 504–517, https://doi.org/10.1093/eurheartj/ehae803
Close - Share Icon Share
Abstract
The Fourth Universal Definition of Myocardial Infarction recommends a classification based on aetiology, in recognition that the underlying pathophysiology of myocardial infarction influences the approach to investigation and treatment. Type 1 myocardial infarction occurs due to atherosclerotic plaque rupture with thrombosis, whereas type 2 myocardial infarction occurs due to an imbalance in myocardial oxygen supply or unmet need in myocardial oxygen demand, without atherothrombosis, usually in the context of another acute illness. In this state-of-the-art review, the diagnosis, investigation, and treatment of patients with type 2 myocardial infarction are considered, with general advice for clinical practice and a consideration of future research directions.
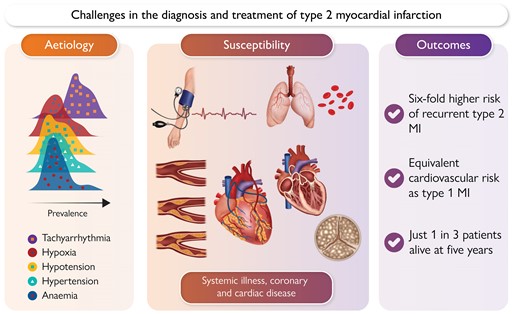
Challenges in the diagnosis and treatment of type 2 myocardial infarction relate to the multiple, often competing aetiologies and underlying disease susceptibility—both of which contribute to an integrated risk of future cardiovascular events. MI, myocardial infarction.
Introduction
Established strategies for the diagnosis and treatment of type 1 myocardial infarction (MI) due to atherosclerotic plaque rupture have led to improvements in both short- and long-term survival. However, it is recognized that MI can occur without atherothrombosis, in acute conditions that result in an imbalance in myocardial oxygen supply or an unmet increase in myocardial oxygen demand. Multiple causes of supply–demand imbalance exist and to date, no approaches to investigation or treatments have been shown to improve outcomes. Here, we explore the underlying mechanisms of type 2 MI, their relationship with future risk, and explore potential strategies for investigation and treatment that could plausibly improve clinical outcomes for a group of patients who are often not given priority.
Identification of myocardial injury and infarction
Cardiac troponin is an integral component of the contractile apparatus of the cardiomyocyte, expressed exclusively within the myocardium. When the heart is injured, cardiac-specific isoforms of troponin I (cTnI) or T (cTnT) are released from cardiomyocytes in a time-dependent manner. Both cTnI and cTnT can be detected in the bloodstream using high-sensitivity assays and are recommended for the diagnosis of myocardial injury and infarction, but they are not biologically equivalent.1,2 Each individual assay has a unique normal reference range and 99th centile upper reference limit (URL), which is the recommended diagnostic threshold.3,4 High-sensitivity assays are defined by their ability to detect cTn concentrations in ≥50% of healthy individuals and have a ≤10% coefficient of variation at the 99th centile URL. There are differences in cardiac troponin concentration between healthy men and women, and sex-specific 99th centiles are recommended for use with high-sensitivity assays to improve performance.5–7 It is essential that clinicians are familiar with the assay in use at their institution and its performance at low-level concentrations, particularly if applying a rapid rule-out algorithm applying decision thresholds below the 99th centile.8,9
Acute myocardial injury is defined as a rise and/or fall in cardiac troponin on serial testing, with at least one value above the 99th centile URL.5 This is a descriptive term, which does not indicate the mechanism of injury and is analogous to acute kidney injury or acute liver injury. If cardiac troponin is above the 99th centile URL but no acute rise and/or fall in cardiac troponin is detected on serial testing, the term chronic myocardial injury is applied. The definition of a rise and/or fall is pragmatic, with a >50% relative change said to exceed analytical and biological variation where the initial concentration is ≤99th centile and a >20% relative change significant where the initial concentration is >99th centile.10,11 Whilst absolute changes appear to have superior diagnostic performance compared to relative changes, these are assay specific.12 It can be difficult to identify changing concentrations if patients present late after onset of symptoms as cardiac troponin takes several days to normalize after an acute injury, or if samples are obtained around peak release.13 Contemporary sensitive assays are still used in some settings, but it is important to note that they may lack precision around the 99th centile and accurate identification of serial change may be challenging. Whilst the 99th centile is a the recommended diagnostic threshold for MI and its use ensures similar standards for care across the world, there is a continuous relationship between cardiac troponin concentration and future cardiovascular risk in patients who have measurable values below the 99th centile.8,14
Definition of myocardial injury and infarction
The Fourth Universal Definition defines an MI as an acute myocardial injury with clinical evidence of acute myocardial ischaemia defined as either symptoms, new changes on the electrocardiogram (ECG) including pathological Q-waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischaemic aetiology.5 Cardiac troponin elevation alone is not specific for MI, irrespective of the absolute or relative change in concentration on serial measurement or the maximal concentration.15 Type 1 MI occurs due to coronary atherosclerotic plaque rupture or erosion with intraluminal thrombosis, and type 2 MI occurs without atherothrombosis, where there is evidence of a reduction in myocardial oxygen supply or an unmet increase in myocardial oxygen demand. When an acute rise and/or fall in cardiac troponin is detected without evidence of ischaemia, the term acute non-ischaemic myocardial injury is applied.16
Aetiology and prevalence of type 2 myocardial infarction
Type 2 MI can occur as a result of an acute systemic illness or cardiac condition or due to an acute coronary pathology other than atherothrombosis,5 but frequently these patients share few characteristics. A 35-year-old with a spontaneous coronary artery dissection occurring during pregnancy, a 65-year-old with MI due to coronary embolism in the context of atrial fibrillation, and an 85-year-old with multi-vessel obstructive coronary artery disease and MI due to profound hypoxia from pneumonia would all be classified as type 2 MI. Such heterogeneity may have inhibited the research and clinical community in developing and evaluating pathways for the investigation and treatment of type 2 MI. Similarly, this complexity can make it challenging for patients to understand their condition and associated prognosis (Graphical Abstract).
The most frequent cause of type 2 MI is tachyarrhythmia, which can be due to a primary cardiac rhythm disturbance or secondary to another acute illness. The remainder of causes may be considered as systemic, relating to hypertension, hypoxaemia, hypotension, or anaemia (Figure 1). Physiological stressors may arise due to critical illness, severe sepsis, haemorrhage, and pulmonary embolism among other causes. It is recognized that in many patients, multiple causative aetiologies are present and this has been shown to be associated with a worse prognosis. Myocardial oxygen supply mismatch may also occur in the context of direct cardiac toxicity, such as cyanide poisoning. Studies that report the most common causes of type 2 MI are summarized in Figure 2. Primary coronary causes of type 2 MI include vasospasm, coronary embolism, or spontaneous coronary artery dissection. These events are less frequently reported in studies of type 2 MI, and patients may present with ST-segment elevation.
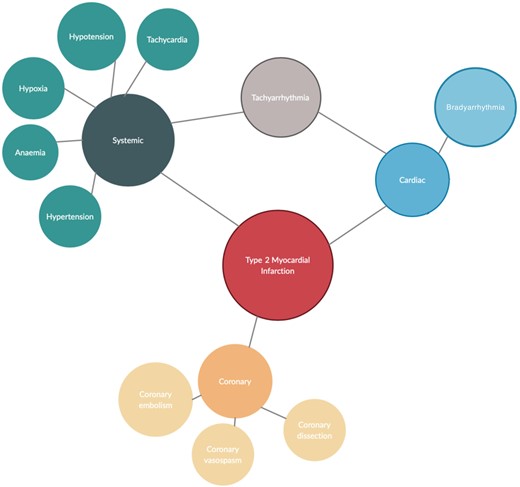
Aetiology of type 2 myocardial infarction. Type 2 myocardial infarction is a descriptive term encompassing a number of different aetiologies. Aetiologies may be stratified as systemic, cardiac, or coronary mechanisms. Patients with these mechanisms may share characteristics, and this might be helpful to guide further investigation and treatment
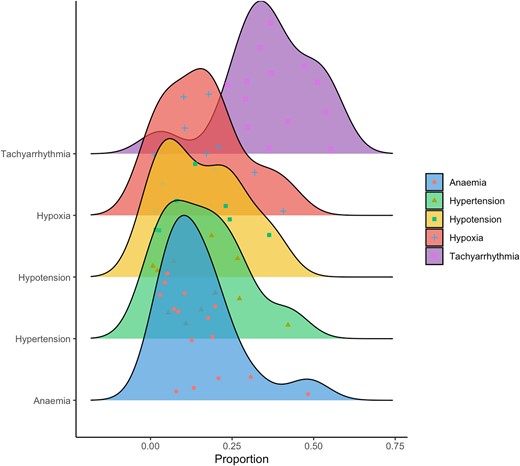
Distribution of type 2 myocardial infarction by aetiology. Density plot illustrating prevalence of type 2 myocardial infarction by aetiology with individual data point for each study cohort and area under the curve representing total number of patients17–33
Widespread adoption of more sensitive cardiac troponin assays has led to increased recognition of myocardial injury and type 2 MI in practice.17,34 Type 2 MI is more common in older females and in those with co-morbidities such as hypertension, hyperlipidaemia, diabetes mellitus, previous coronary revascularization, and previous heart failure.18–21,35–38 Estimates of the prevalence of type 2 MI vary widely depending on the setting (emergency department, hospitalized patients, or the coronary care unit), the method of identification (consented or consecutive), and differences in the approach to defining the diagnosis (adjudication or hospital coding). In a cohort of consecutive patients presenting to an emergency department with suspected acute coronary syndrome in Scotland, the prevalence of type 2 MI was 12.3%.34 Whereas in patients admitted to hospital, the prevalence of type 2 MI varies from 2% to 37%.6,39–45 Type 2 MI is as prevalent as type 1 MI in those over the age of 75 years.39 Importantly, even in registry-based studies with standard data collection procedures, significant variation in prevalence has been observed from .2% to 13.0% in the SWEDEHEART registry.19,46 The variation in prevalence observed between studies may be compounded by differences in application of the diagnostic criteria during adjudication, which enables the diagnosis to be made using symptoms of myocardial ischaemia alone. This can be subjective in patients unwell with another cardiac condition.
As cardiac troponin is generally only measured in patients with the highest pre-test probability where the clinician reasonably suspects myocardial injury or ischaemia, the true prevalence of myocardial injury and type 2 MI may be higher. In the CHARIOT study, cardiac troponin was measured in all hospitalized patients where serum blood samples were obtained.47 The prevalence of myocardial injury in patients where cardiac troponin was measured without a clinical indication was 4.6% (836/18 171). Diagnostic coding was used to identify and exclude patients with a clinical diagnosis of MI, and whilst the diagnosis was not adjudicated, it is likely the majority of patients with elevated cardiac troponin were unrecognized acute non-ischaemic myocardial injury or type 2 MI. Similar observations were reported in a multi-centre analysis comparing selected vs. unselected testing in the UK and the USA, respectively, demonstrating a higher prevalence of type 2 MI or acute myocardial injury with an unselected approach.48 Judicious testing is advocated to avoid diagnostic uncertainty and to minimize the risk of misdiagnosis and inappropriate treatment of acute coronary syndrome, such as when type 2 MI has occurred as a consequence of anaemia from acute bleeding. However, the latest European Society of Cardiology guidelines for the evaluation of patients undergoing non-cardiac surgery recommend serial measurement of cardiac troponin before and after surgery.49 Given there is limited evidence to guide treatment of patients with myocardial injury after non-cardiac surgery,50 and no evidence to guide treatment of patients with type 2 MI, it is not clear how this advice may be consistently implemented in practice.
Clinical outcomes and competing risks
In patients with type 2 MI, outcomes are generally poor.19,20,22,23,45,51–56 There is a high early event rate at 30 days, which may relate to the primary illness rather than the secondary insult.24 However, this excess risk persists beyond the index event, and at 5 years, only around one-third of patients are alive.34,57 It is often stated that the excess in all-cause mortality observed after type 2 MI is attributable to age and co-morbidity, and not related to cardiovascular disease. However, in a multi-centre randomized controlled trial enrolling 48 282 consecutive unselected patients with suspected acute coronary syndrome in whom the diagnosis was classified according to the Universal Definition, cause-specific mortality was explored.40 At 1 year, deaths from a non-cardiovascular cause occurred in 12% of patients with type 2 MI and 5% of patients with type 1 MI, the latter of which was comparable to those without myocardial injury. Despite an excess in non-cardiovascular events in those with type 2 MI, the absolute rates of MI or death from a cardiovascular cause were comparable, affecting 17% and 14% of patients with type 1 and type 2 MI, respectively. Even after adjustment, the risk of cardiovascular events in type 2 MI was elevated with a cause-specific hazard ratio (HR) of 3.50 [95% confidence interval (CI) 2.94–4.15] compared to patients without myocardial injury.34 Similar observations have been made during follow-up of 63 479 patients from the SWEDEHEART cohort.56,58 Patients with type 2 MI were at higher risk of major adverse cardiovascular events (adjusted HR 1.28, 95% CI 1.22–1.34) at 2.8 years compared to those with type 1 MI. A further cohort study of patients with type 1 and type 2 MI recruited within Olmsted County, MN, identified 1345 and 1022 patients with type 1 and type 2 MI. At 5 years, there were 31% and 59% deaths from any cause, of which 18% and 21% were due to cardiovascular disease, in type 1 or type 2 MI, respectively.57
The concept of competing risk of non-cardiovascular events is fundamental to our interpretation of future cardiovascular event rates in this population. As fewer patients with type 2 MI are alive due to the competing risk of non-cardiovascular death, fewer have the potential to experience a cardiovascular event, and one would therefore anticipate a much lower incidence in longitudinal follow-up if the underlying risks were comparable. As the observed cardiovascular event rates are similar, it is likely future cardiovascular risk is at least as high in type 2 as in type 1 MI.
Aetiology of supply or demand imbalance and clinical outcomes
The aetiology of supply or demand imbalance is associated with future prognosis in patients with type 2 MI. Patients with type 2 MI related to tachyarrhythmia have a more favourable prognosis, comparable to those with type 1 MI, whereas patients with type 2 MI related to hypoxia, anaemia, or hypotension have the worst prognosis. In fully adjusted analyses, those with hypoxia and anaemia have a two-fold increased risk of death from any cause at 1 year relative to those with type 1 MI.25 Efforts should be made to understand the aetiology of type 2 MI as it may inform prognosis as well as treatment.
Risk assessment for cardiovascular disease
Risk factors for the development of type 1 and type 2 MI are comparable. In the CASABLANCA cohort, 1251 patients with peripheral vascular disease were followed up for a median of 3.4 years. Patients who developed an incident type 1 or type 2 MI had a similar prevalence of hypertension (82% vs. 86%), coronary artery disease (76% vs. 70%), and prior MI (39% vs. 34%), but differences in known heart failure (15% vs. 36%), previous percutaneous coronary intervention (52% vs. 42%), and previous coronary artery bypass grafting (49% vs. 31%), respectively.36 In a secondary analysis of the High-STEACS trial, 924 and 407 incident type 1 and type 2 MI events occurred during 1 year of follow-up, respectively. Risk factors for the development of type 1 and type 2 MI were similar, with age, hyperlipidaemia, diabetes mellitus, abnormal renal function, and coronary artery disease predictive for both.59 Although several previous studies demonstrate type 2 MI is more prevalent in women,17–21,39,60 in this analysis, sex was not associated with MI subtype. The strongest predictor of future type 2 MI was a prior history of type 2 events, with an over six-fold increased risk (HR 6.18, 95% CI 4.70–8.12). This similarity in cardiovascular risk profile between MI subtypes suggests coronary artery disease is likely to be an important contributor to risk of both type 1 and type 2 MI events, respectively.
Risk stratification and identification of cardiovascular disease
Accurate risk stratification for cardiovascular disease in patients with type 2 MI requires an understanding of the aetiology, previous history, risk factors, and an assessment of the individuals’ likely ischaemic threshold. For example, a younger patient without co-morbidity and with no coronary artery disease would require a prolonged episode of extreme tachycardia to cross the ischaemic threshold and cause myocardial injury, which may be a magnitude lower in an older patient with underlying obstructive coronary artery disease experiencing even a brief episode of moderate tachycardia (Figure 3).43 It is likely that multiple competing issues including illness severity, co-morbidity, blood pressure, haemoglobin, atherosclerotic burden, subclinical obstructive coronary disease, left ventricular function, and the presence of valvular heart disease all contribute to an integrated risk of myocardial oxygen supply or demand imbalance. Such variability is one of the primary challenges in patients with type 2 MI, and careful clinical assessment is required to delineate if an identified pathology such as severe aortic valve stenosis is the responsible aetiology, or whether it has increased a patient’s susceptibility to type 2 MI that was triggered by another event (Figure 4).
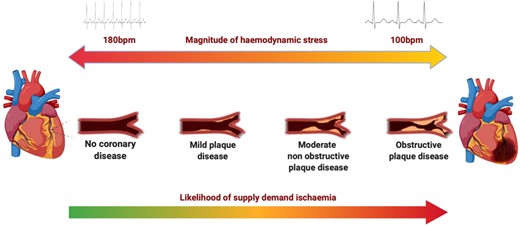
Evaluation of the ischaemic threshold. The magnitude of haemodynamic stress and burden of coronary atheroma influence the likelihood of supply–demand ischaemia and type 2 myocardial infarction. A patient without coronary artery disease requires a significant haemodynamic stressor to result in supply or demand imbalance compared to a patient with obstructive coronary artery disease. This illustration considers coronary disease in isolation for simplicity, but in practice, multiple competing aetiologies contribute
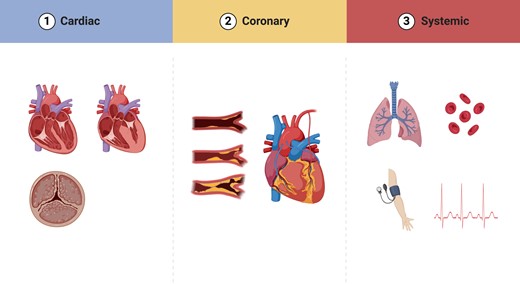
Susceptibility to type 2 myocardial infarction. Multiple factors contribute to an individual patient’s susceptibility to myocardial oxygen supply or demand imbalance. Patient, coronary, and structural factors are all important, some of which may be modifiable and could plausibly reduce the likelihood of recurrent events
Risk stratification tools are available for use in patients with type 2 MI. The GRACE 2.0 score was derived in patients with acute coronary syndrome prior to the introduction of high-sensitivity cardiac troponin assays and the classification of MI by aetiology.61,62 The GRACE score has moderate discrimination for future MI or death at 1 year in patients with type 2 MI [area under the curve (AUC) .73, 95% CI .70–.77).63 The TARRACO score is a bespoke tool derived in patients with type 2 MI and myocardial injury,64 with moderate discrimination for future adverse cardiovascular events and mortality (AUC .74, 95% CI .70–.79). However, in a recent comparison of the GRACE and TARRACO scores in patients with type 2 MI, GRACE was predictive of all-cause mortality (AUC .70, 95% CI .63–.77), but TARRACO was not (AUC .52, 95% CI .46–.58).65
The T2-risk score was derived in patients with type 2 MI using cardiac troponin concentrations measured with a high-sensitivity assay and clinical variables including age, sex, renal function, a history of ischaemic heart disease, previous MI, heart failure hospitalization, stroke, diabetes mellitus, ischaemia on 12-lead ECG, and anaemia. When compared to GRACE 2.0, this tool demonstrated superior discrimination and calibration for future MI and death at 1 year (AUC .76, 95% CI .73–.79). External validation in a multi-centre cohort study and in an unselected consecutive patient cohort study demonstrated consistent performance in both populations.66 Whether the T2-risk score is helpful in practice requires prospective evaluation.
Blood biomarkers and pathophysiology of type 2 myocardial infarction
Whilst maximal cardiac troponin concentrations tend to be higher after a type 1 compared with type 2 MI, no decision threshold has sufficient discrimination to be used in practice.15 It is hypothesized that cardiac troponin may be released in patients without cardiomyocyte necrosis. Cardiomyocytes undergo mechanical stretch in response to pressure or volume overload, and this may trigger activation of proteases associated with intracellular degradation and release of troponin fragments from the cytosol.67 Tachycardia may stimulate stress-responsive integrins, triggering release of intact cardiac troponin I from viable cardiomyocytes in the absence of necrosis.68 Furthermore, troponin release has been demonstrated in vivo during nuclear perfusion imaging, with peak troponin concentration associated with the extent of myocardial ischaemia.69
Novel markers of cardiomyocyte necrosis may provide further insights. Following cellular necrosis, fragments of genomic cell-free DNA are released and briefly circulate prior to hepatic clearance. A cardiomyocyte-specific cell-free DNA assay targeting the FAM101A locus has been developed and validated in patients with ST-segment elevation MI. This assay had excellent discrimination for MI and was strongly correlated with maximal cardiac troponin concentration (R .80).70 When evaluated in healthy individuals who had completed a half-marathon or marathon, despite substantial elevation in cardiac troponin I concentrations (median 85 ng/L, range 7–1900 ng/L), cardiomyocyte cell-free DNA concentrations remained low and did not correlate well with cardiac troponin (R = .37). Unlike in MI, the extent of cardiomyocyte necrosis was insufficient to explain the magnitude of cardiac troponin release, perhaps supporting the hypothesis that reversible injury has occurred.
A novel assay to quantify intact ‘long-form’ cTnT has been developed, which is thought to be released exclusively following cardiomyocyte necrosis. This contrasts to the current commercially available high-sensitivity cTnT assay that may detect small heavily cleaved fragments of cTnT. It is thought that fragments may explain the high prevalence of chronic myocardial injury detected with this assay. In a small study, the ratio of long-form to total cTnT (including fragments) had excellent discrimination for patients with Non-ST segment elevation myocardial infarction compared to chronic myocardial injury (AUC .96, 95% CI .89–1.00).71 A novel highly sensitive immunoassay with a 28-fold lower limit of detection has now been developed, with improved precision and excellent discrimination between NSTEMI and chronic myocardial injury (AUC .99, 95% CI .97–100). Whether these observations could be used to discriminate patients with acute non-ischaemic myocardial injury or type 2 MI in the context of supply or demand imbalance from those with type 1 MI is unknown and requires prospective evaluation, but such assays remain pre-clinical and are yet to be translated to the clinical laboratory.72 In contrast to cardiac troponin T, there do not appear to be significant differences in the presence of free, intact, and complex cardiac troponin I concentrations between patients with type 1 MI and those with alternative causes of troponin elevation including type 2 MI.73 Notably, all studies of troponin fragments may be affected by time course degradation. The optimal study design would require serial coronary sinus sampling early after symptom onset in patients with type 1 and type 2 MI. Whilst this might be achievable in type 1 MI where there is usually a clear symptom onset time, in type 2 MI, this would be highly challenging and models of supply or demand ischaemia may be required.74
Panels of blood biomarkers that reflect different pathophysiological processes may also aid in the discrimination of type 1 from type 2 MI, with markers of endothelial or microvascular dysfunction demonstrating moderate discrimination, albeit they did not exceed the performance of cardiac troponin alone.75 A further study including a panel of 29 candidate biomarkers including apolipoprotein A-II, NT-proBNP, copeptin, and cTnI had good discrimination between type 1 and type 2 MI (AUC .82), but this requires prospective external validation and the clinical utility is uncertain.76
Coronary artery disease
In most patients, the diagnosis of type 2 MI is made based on clinical assessment alone and confirmatory diagnostic testing is not undertaken. However, there are undoubtedly patients in whom it is not possible to distinguish between type 1 and type 2 MI without coronary imaging. Whilst the Universal Definition does not mandate coronary imaging, even in patients with type 1 MI, if there is clinical ambiguity as to whether atherothrombosis has occurred, invasive coronary angiography should be performed.5 This may identify plaque rupture, erosion, atherothrombosis, embolism, vasospasm, dissection, stable coronary artery disease, or if unclear, may provide the opportunity for further evaluation with optical coherence tomography (OCT) or intravascular ultrasound (IVUS).
In the DEMAND-MI (DEtermining the Mechanism of myocardial injury AND role of coronary disease in type 2 Myocardial Infarction) prospective observational cohort study, consecutive patients with myocardial injury were screened to identify those with a clinical diagnosis of type 2 MI according to the Universal Definition.77 Patients had invasive angiography with intravascular plaque imaging where available, or computed tomography (CT) coronary angiography and cardiac magnetic resonance imaging (MRI) or echocardiography. In 1 in 20 patients, coronary imaging demonstrated the clinical diagnosis of type 2 MI was incorrect, with angiography identifying evidence of plaque rupture or thrombosis (type 1 or 4b MI). In others, cardiac imaging identified a clear alternative pathology including Takotsubo cardiomyopathy and myocarditis, with immediate implications for further management. Similar findings have been observed in a retrospective case review of patients with type 2 MI.26 Of 27% of patients with a clinical diagnosis of type 2 MI who underwent invasive coronary angiography, evidence of plaque rupture was demonstrated in one in three. This is likely to be an overestimate of the true rates of misclassification as unlike in the DEMAND-MI study, patients were selected for coronary angiography on the basis of clinical assessment, and it is likely the pre-test probability of coronary disease was higher.
In DEFINE-TYPE 2 MI, McCarthy et al.78 prospectively enrolled patients with type 2 MI for CT coronary angiography with CT with fractional flow reserve. The prevalence of coronary artery plaque disease was 92%, which was obstructive in 42% and previously unrecognized and untreated in almost all. These findings are consistent with DEMAND-MI, where the prevalence of any coronary artery disease was 68%, which was obstructive in 30% of patients. Across all studies, there is variability in the reported prevalence of coronary artery disease as the design, inclusion and exclusion criteria, and definitions differ.21,27,35,38,45,51,55,79 Importantly, in all studies of cardiac imaging in type 2 MI, there is some selection bias. In DEMAND-MI, to minimize participant risk, patients with significant renal or hepatic injury or frailty were excluded, and it is likely that a lower risk population were recruited. The importance of selection bias in registry-based studies should also be considered, as the pre-test probability for coronary disease in studies of those who undergo invasive angiography based on clinical indication is likely to be a magnitude higher than in unselected patients.36
Which patients should undergo investigation and when?
In the absence of randomized controlled trial evidence to support investigative strategy, we propose a simple decision tree for the evaluation of patients with myocardial ischaemia secondary to oxygen supply or demand imbalance. The aim is to guide initial assessment and investigation to aid diagnosis and identification of significant underlying cardiac pathology that could plausibly benefit from treatment (Figure 5).
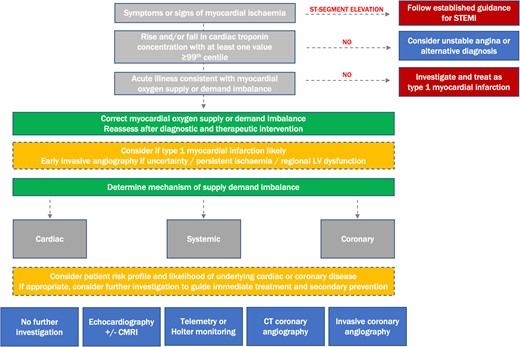
Diagnostic algorithm for the evaluation of patients with myocardial ischaemia in clinical practice. The clinician determines if a patient has evidence of myocardial ischaemia, either based on symptoms or electrocardiogram criteria. Where electrocardiogram evidence of ST-segment elevation exists, established pathways are followed. In patients without a rise and/or fall in cardiac troponin, differential diagnoses are considered. In those with evidence of supply or demand imbalance, this is corrected where possible. A reassessment is undertaken to identify if symptoms or signs of ischaemia persist, and the diagnosis of type 1 myocardial infarction considered. A clinical assessment of the mechanism of type 2 myocardial infarction is made, and additional investigations considered on the basis of risk profile, mechanism, and likelihood of cardiac disease to guide immediate treatment and secondary prevention. STEMI, ST-segment elevation myocardial infarction; CMRI, cardiac MRI; CT, computed tomography
Some general principles may be helpful in determining which patients are most likely to benefit from invasive coronary angiography (Figure 6). Firstly, consideration of the magnitude of supply–demand imbalance and its relationship with the time of onset and duration of myocardial ischaemia is important. If myocardial ischaemia only occurs during periods of supply or demand imbalance and resolves after correction, acute plaque rupture is less likely. However, underlying obstructive coronary artery disease is still possible and perhaps more likely if regional changes are present on the ECG. If there is diagnostic uncertainty, or in unstable patients with ongoing evidence of ischaemia, the threshold for early invasive assessment is lower. Invasive angiography can facilitate OCT or IVUS, which can exclude recent atherosclerotic plaque rupture or erosion, and invasive functional assessment can ascertain whether disease is likely to contribute to symptoms or recurrent events. In patients who are stable and likely to have underlying coronary artery disease, we propose anatomical investigation as a first-line strategy. Computed tomography coronary angiography is a low-risk, non-invasive investigation that can identify obstructive coronary disease and allow targeted secondary prevention therapy, which has been shown to improve outcomes in patients with chronic coronary syndromes.80,81 Where prognostically important left main stem or three-vessel coronary artery disease is identified, or in those with a high pre-test probability, invasive coronary angiography should be considered. Deferred invasive coronary angiography may also be considered as a first-line investigation in patients with significant regional ischaemia and a high pre-test probability of obstructive coronary disease.
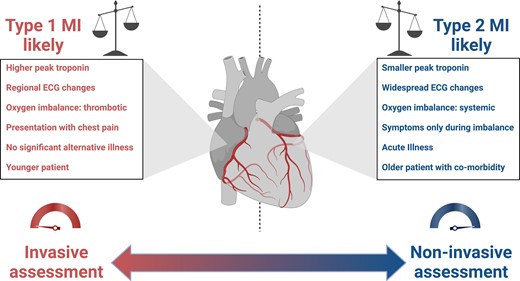
Evaluation of the patient with type 1 or type 2 myocardial infarction. Several factors may guide the clinician in determining whether an initial invasive or non-invasive assessment is appropriate to guide diagnosis and treatment. Where the balance favours a diagnosis of type 1 myocardial infarction, where atherothrombosis has resulted in oxygen imbalance, initial invasive assessment is proposed. MI, myocardial infarction; ECG, electrocardiogram
Non-invasive functional imaging may also be considered, particularly in patients with a contraindication to invasive assessment, and may be both informative and prognostic. In 234 patients with type 2 MI who underwent myocardial perfusion imaging, an abnormal result (defined as a perfusion defect involving ≥5% of left ventricular myocardium) was identified in 58% (136/234), which was associated with an increased risk of death, MI, and late coronary revascularization (adjusted HR 1.45, 95% CI 1.02–2.06).82
The prevalence of left ventricular systolic dysfunction is variable in patients with type 2 MI with a reported frequency from 8% to 51%.17,19,20,24,38,55–57 The ischaemic insult from type 2 MI may cause deterioration in ventricular function or identify patients with prior ventricular dysfunction that may or may not have been previously recognized, presenting an opportunity to initiate or optimize treatment. In DEMAND-MI, 34% of patients were found to have left ventricular systolic dysfunction, which was moderate or severe in 19%.77 Where further investigation is considered appropriate, an echocardiogram is a reasonable first-line investigation. Where image quality is poor or if an underlying cardiomyopathic process is suspected, cardiac MRI can be considered.
In some cases, the diagnosis of type 2 MI may appear obvious, for example where myocardial oxygen supply or demand imbalance is suspected due to severe hypoxia or when acute blood loss has occurred due to gastrointestinal haemorrhage. However, infection and bleeding result in a pro-thrombotic, pro-coagulant state associated with an increased risk of type 1 MI that may persist for several weeks.83 There is therefore a risk to dismissing all presentations with supply or demand imbalance as type 2 MI without careful assessment, as further investigation may reclassify the diagnosis and identify opportunities for treatment (Figure 7).
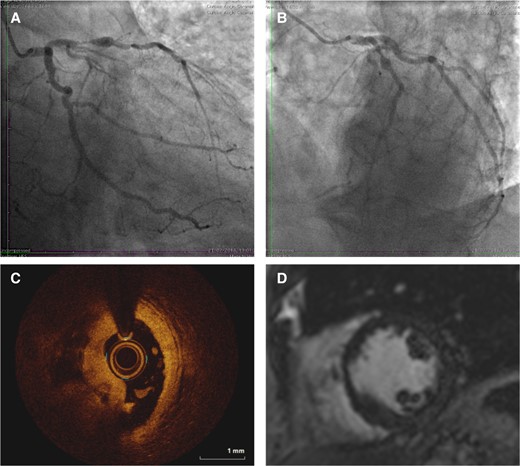
Illustrative case example of patient with clinical diagnosis of type 2 myocardial infarction where the use of invasive coronary angiography and optical coherence tomography led to diagnostic reclassification. A 64-year-old presents with increasing ischaemic sounding chest pain, with fever, breathlessness, and cough for 2 days. The admission observations were heart rate 114, oxygen saturations 93% on 4L, respiratory rate 34, temperature 38.9 °C, and blood pressure 156/78. The electrocardiogram demonstrates a presumed new left bundle branch block. A chest X-ray demonstrates right lower lobe consolidation with a C-reactive protein 160 ng/L. The peak high-sensitivity cardiac troponin I was 6451 ng/L. This was treated as type 2 myocardial infarction. Coronary angiography demonstrated a severe lesion in the proximal left anterior descending artery with collaterals to an occluded right coronary artery (A and B) with intracoronary plaque rupture and thrombosis in the left anterior descending artery on optical coherence tomography (C), and corresponding basal anteroseptal late gadolinium enhancement on cardiac magnetic resonance imaging scan (D). The diagnosis was reclassified as type 1 myocardial infarction. Reproduced with permission from the DEMAND-MI educational resource with identifiable features changed77
There are some patients with type 2 MI in whom further investigation is very unlikely to influence treatment or outcomes, due to advanced age, significant frailty, or co-morbidity with life-limiting illness. Similarly, there are some patients in whom the pre-test probability of identifying underlying cardiac disease is sufficiently low to avoid testing altogether. This threshold for further investigation varies on an individual patient basis, and careful assessment of the clinical presentation, aetiology, and magnitude of supply–demand imbalance is required. This assessment should include identification of cardiovascular risk factors, clinical examination for evidence of left ventricular dysfunction, or valvular heart disease and risk assessment for coronary artery disease.
Treatment
No dedicated trials focused exclusively on treatment strategies in patients with type 2 MI have been undertaken. The following general principles may be helpful, with a summary of specific considerations by aetiology of type 2 MI provided in Table 1.
Aetiology of type 2 myocardial infarction and general principles to guide further investigation and treatment
| Aetiology of type 2 MI . | Specific management principles by aetiology . | General principles for all type 2 MI . |
|---|---|---|
| Tachyarrhythmia | Ongoing ischaemia may require intervention with chemical or DC cardioversion Identify if episode is secondary to an acute illness, and correct any reversible triggers Consider definitive treatment (anti-arrhythmic therapy or ablation) based on likelihood of recurrence Determine if anticoagulation is indicated | Always consider differential diagnosis that includes type 1 myocardial infarction Where there is diagnostic uncertainty, consider inpatient invasive coronary angiography and intravascular imaging if required to identify plaque rupture or thrombosis Evidence of regional ischaemia in type 2 MI suggests underlying obstructive coronary disease Consider evaluation for coronary artery disease following resolution of acute stressor, this may be undertaken with CTCA with timing at clinician discretion Consider risk stratification for underlying LV impairment with BNP and echocardiography Secondary prevention with aspirin and statin should be considered following risk stratification |
| Hypertension | Severe hypertension can be a cause or a result of ischaemic chest discomfort Look for other evidence of secondary end-organ dysfunction suggestive of persistent severe hypertension (proteinuria, papilloedema, and left ventricular hypertrophy) If hypertensive crisis confirmed, consider cautious blood pressure reduction | |
| Hypotension | Treat underlying illness and support blood pressure to reduce myocardial ischaemia | |
| Hypoxia | Treat underlying illness and support oxygenation to reduce myocardial ischaemia | |
| Anaemia | Identify evidence of active occult bleeding Consider blood transfusion if ongoing ischaemia | |
| Coronary | Vasospasm: consider provocation testing if diagnosis uncertain and targeted vasodilator therapy Spontaneous coronary artery dissection: consider additional screening for fibromuscular dysplasia Embolism: consider prolonged rhythm monitoring for atrial fibrillation and bubble contrast echocardiography to identify PFO/ASD |
| Aetiology of type 2 MI . | Specific management principles by aetiology . | General principles for all type 2 MI . |
|---|---|---|
| Tachyarrhythmia | Ongoing ischaemia may require intervention with chemical or DC cardioversion Identify if episode is secondary to an acute illness, and correct any reversible triggers Consider definitive treatment (anti-arrhythmic therapy or ablation) based on likelihood of recurrence Determine if anticoagulation is indicated | Always consider differential diagnosis that includes type 1 myocardial infarction Where there is diagnostic uncertainty, consider inpatient invasive coronary angiography and intravascular imaging if required to identify plaque rupture or thrombosis Evidence of regional ischaemia in type 2 MI suggests underlying obstructive coronary disease Consider evaluation for coronary artery disease following resolution of acute stressor, this may be undertaken with CTCA with timing at clinician discretion Consider risk stratification for underlying LV impairment with BNP and echocardiography Secondary prevention with aspirin and statin should be considered following risk stratification |
| Hypertension | Severe hypertension can be a cause or a result of ischaemic chest discomfort Look for other evidence of secondary end-organ dysfunction suggestive of persistent severe hypertension (proteinuria, papilloedema, and left ventricular hypertrophy) If hypertensive crisis confirmed, consider cautious blood pressure reduction | |
| Hypotension | Treat underlying illness and support blood pressure to reduce myocardial ischaemia | |
| Hypoxia | Treat underlying illness and support oxygenation to reduce myocardial ischaemia | |
| Anaemia | Identify evidence of active occult bleeding Consider blood transfusion if ongoing ischaemia | |
| Coronary | Vasospasm: consider provocation testing if diagnosis uncertain and targeted vasodilator therapy Spontaneous coronary artery dissection: consider additional screening for fibromuscular dysplasia Embolism: consider prolonged rhythm monitoring for atrial fibrillation and bubble contrast echocardiography to identify PFO/ASD |
Aetiology of type 2 myocardial infarction and general principles to guide further investigation and treatment
| Aetiology of type 2 MI . | Specific management principles by aetiology . | General principles for all type 2 MI . |
|---|---|---|
| Tachyarrhythmia | Ongoing ischaemia may require intervention with chemical or DC cardioversion Identify if episode is secondary to an acute illness, and correct any reversible triggers Consider definitive treatment (anti-arrhythmic therapy or ablation) based on likelihood of recurrence Determine if anticoagulation is indicated | Always consider differential diagnosis that includes type 1 myocardial infarction Where there is diagnostic uncertainty, consider inpatient invasive coronary angiography and intravascular imaging if required to identify plaque rupture or thrombosis Evidence of regional ischaemia in type 2 MI suggests underlying obstructive coronary disease Consider evaluation for coronary artery disease following resolution of acute stressor, this may be undertaken with CTCA with timing at clinician discretion Consider risk stratification for underlying LV impairment with BNP and echocardiography Secondary prevention with aspirin and statin should be considered following risk stratification |
| Hypertension | Severe hypertension can be a cause or a result of ischaemic chest discomfort Look for other evidence of secondary end-organ dysfunction suggestive of persistent severe hypertension (proteinuria, papilloedema, and left ventricular hypertrophy) If hypertensive crisis confirmed, consider cautious blood pressure reduction | |
| Hypotension | Treat underlying illness and support blood pressure to reduce myocardial ischaemia | |
| Hypoxia | Treat underlying illness and support oxygenation to reduce myocardial ischaemia | |
| Anaemia | Identify evidence of active occult bleeding Consider blood transfusion if ongoing ischaemia | |
| Coronary | Vasospasm: consider provocation testing if diagnosis uncertain and targeted vasodilator therapy Spontaneous coronary artery dissection: consider additional screening for fibromuscular dysplasia Embolism: consider prolonged rhythm monitoring for atrial fibrillation and bubble contrast echocardiography to identify PFO/ASD |
| Aetiology of type 2 MI . | Specific management principles by aetiology . | General principles for all type 2 MI . |
|---|---|---|
| Tachyarrhythmia | Ongoing ischaemia may require intervention with chemical or DC cardioversion Identify if episode is secondary to an acute illness, and correct any reversible triggers Consider definitive treatment (anti-arrhythmic therapy or ablation) based on likelihood of recurrence Determine if anticoagulation is indicated | Always consider differential diagnosis that includes type 1 myocardial infarction Where there is diagnostic uncertainty, consider inpatient invasive coronary angiography and intravascular imaging if required to identify plaque rupture or thrombosis Evidence of regional ischaemia in type 2 MI suggests underlying obstructive coronary disease Consider evaluation for coronary artery disease following resolution of acute stressor, this may be undertaken with CTCA with timing at clinician discretion Consider risk stratification for underlying LV impairment with BNP and echocardiography Secondary prevention with aspirin and statin should be considered following risk stratification |
| Hypertension | Severe hypertension can be a cause or a result of ischaemic chest discomfort Look for other evidence of secondary end-organ dysfunction suggestive of persistent severe hypertension (proteinuria, papilloedema, and left ventricular hypertrophy) If hypertensive crisis confirmed, consider cautious blood pressure reduction | |
| Hypotension | Treat underlying illness and support blood pressure to reduce myocardial ischaemia | |
| Hypoxia | Treat underlying illness and support oxygenation to reduce myocardial ischaemia | |
| Anaemia | Identify evidence of active occult bleeding Consider blood transfusion if ongoing ischaemia | |
| Coronary | Vasospasm: consider provocation testing if diagnosis uncertain and targeted vasodilator therapy Spontaneous coronary artery dissection: consider additional screening for fibromuscular dysplasia Embolism: consider prolonged rhythm monitoring for atrial fibrillation and bubble contrast echocardiography to identify PFO/ASD |
Immediate
In patients who have a clear trigger for type 2 MI and who are thought to be at low risk of underlying cardiovascular disease, no treatment may be required other than correcting the underlying reason for supply–demand imbalance to reduce the risk of recurrent myocardial ischaemia. This may include temporarily withholding medication that could exacerbate supply–demand imbalance, such as anti-hypertensive therapy, and attempting to restore normal physiology through intra-venous fluid resuscitation, oxygen supplementation, or blood transfusion to limit further ischaemia.
In-hospital
For patients who have recurrent symptoms of myocardial ischaemia, particularly those with a substantial increase in cardiac troponin concentration, clear changes on the ECG, or a regional wall motion abnormality on echocardiography, invasive coronary angiography may be indicated to identify those with obstructive coronary disease or plaque rupture. In patients found to have evidence of atherothrombosis, treatment should follow clinical practice recommendations for type 1 MI.84 In those where the diagnosis of type 2 MI is confirmed and coronary artery disease is identified, single antiplatelet and lipid-lowering therapy should be considered. No prospective randomized clinical trials have evaluated coronary revascularization in patients with type 2 MI, with a wide variety in reported rates of revascularization from 0% to 51%.17–20,28,29,39,85 The ACT-2 trial will evaluate the role of coronary imaging, with revascularization decisions at the discretion of the attending cardiologist.86 Patients with symptoms of stable angina have a clear indication for angiography and revascularization should be considered.84 Patients with left ventricular systolic dysfunction and acute or chronic heart failure should be managed according to established guidelines.87 In the context of supply–demand mismatch, it is unlikely all evidence-based therapies could be safely commenced in-hospital. Once optimal diuresis has been obtained, therapies that do not have a blood pressure-lowering effect such as SGLT2 inhibitors would be a reasonable first-line strategy, with additional goal-directed medical therapy according to clinical guidelines.
Research requirements
For clinical trials to be successful in type 2 MI, the design will need to be pragmatic, with minimal exclusion criteria, and involve multiple centres. Given this heterogeneous patient population, it is unlikely a single intervention will be effective and complex interventions may be needed. Although the risk of future cardiovascular events including recurrent type 2 MI is high, it should be acknowledged that the potential for adverse unintended consequences of treatment such as bleeding may be greater, particularly in those who are older with multi-morbidity. Furthermore, the competing risk of non-cardiovascular events may limit any potential treatment benefit. Strategies to determine which patients stand to benefit from investigation and treatment require exploration (Table 2).
| Determining the clinical utility of diagnostic imaging . | Rationale . |
|---|---|
| Intravascular imaging (optical coherence tomography or intravascular ultrasound) | To define the frequency of misclassification in those with plaque rupture or erosion with coronary thrombosis. To identify high-risk plaque phenotypes that might increase risk of recurrent myocardial infarction. To systematically evaluate and differentiate subtypes of spontaneous coronary artery dissection. |
| CT coronary angiography | To determine diagnostic performance in type 2 myocardial infarction and ability to discriminate type 1 and type 2 myocardial infarction. To define the prevalence of high-risk plaque phenotypes (calcific plaque, non-calcific plaque, and low-attenuation plaque). |
| Cardiac MRI | To define the frequency of late gadolinium enhancement in type 2 myocardial infarction. To confirm the diagnosis in those with inducible ischaemia and the association with recurrent events. To determine if the prevalence of inducible ischaemia differs in those with type 2 myocardial infarction and acute myocardial injury. |
| Microvascular assessment | To determine the prevalence coronary microvascular disease. |
| Coronary vasospasm | To determine the diagnostic utility of provocation testing for vasomotor dysfunction. |
| Determining the clinical utility of diagnostic imaging . | Rationale . |
|---|---|
| Intravascular imaging (optical coherence tomography or intravascular ultrasound) | To define the frequency of misclassification in those with plaque rupture or erosion with coronary thrombosis. To identify high-risk plaque phenotypes that might increase risk of recurrent myocardial infarction. To systematically evaluate and differentiate subtypes of spontaneous coronary artery dissection. |
| CT coronary angiography | To determine diagnostic performance in type 2 myocardial infarction and ability to discriminate type 1 and type 2 myocardial infarction. To define the prevalence of high-risk plaque phenotypes (calcific plaque, non-calcific plaque, and low-attenuation plaque). |
| Cardiac MRI | To define the frequency of late gadolinium enhancement in type 2 myocardial infarction. To confirm the diagnosis in those with inducible ischaemia and the association with recurrent events. To determine if the prevalence of inducible ischaemia differs in those with type 2 myocardial infarction and acute myocardial injury. |
| Microvascular assessment | To determine the prevalence coronary microvascular disease. |
| Coronary vasospasm | To determine the diagnostic utility of provocation testing for vasomotor dysfunction. |
| Determining the effectiveness of therapeutic strategies . | Rationale . |
|---|---|
| Antiplatelet therapy | To determine the effectiveness of single or dual antiplatelet therapy in the reduction of future events in patients with type 2 myocardial infarction. |
| Lipid-lowering therapy | The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab reduced the incidence of type 1 (HR .87, 95% CI .77–.99) and type 2 (HR .77, 95% CI .61–.97) myocardial infarction during follow-up (ODYSSEY OUTCOMES trial),29,85,88 demonstrating proof of principle that lipid-lowering therapy may be effective at reducing both type 1 and type 2 myocardial infarction. |
| SGLT2 inhibitors | Empagliflozin was associated with a reduction in future type 1 (RR .79, 95% CI .61–1.04) and type 2 myocardial infarction events (RR .67, 95% CI .41–1.10) at 3.1 years in patients with type 2 diabetes mellitus and cardiovascular disease (EMPA-REG OUTCOME trial).86,89 |
| Coronary revascularization | PCI or CABG compared to medical therapy was associated with a lower mortality in 268 850 patients with type 2 myocardial infarction in a propensity-matched analysis (3.9% vs. 5.5%, OR .70, 95% CI .59–.84, P < .001).90 Prospective studies that minimize the risk of confounding are needed. |
| Blood transfusion in patients with anaemia | The MINT trial demonstrated no difference between a restrictive or liberal transfusion strategy in patients with myocardial infarction (1.15, 95% CI .99–1.34, P = .07). A subgroup analysis suggested a potential signal for harm from a restrictive strategy in type 1 MI (RR 1.32, 95% CI 1.04–1.67) and no difference in type 2 MI (RR 1.05, 95% CI .85–1.29), but this comparison was underpowered.91 |
| GLP-1 agonists | The glucagon-like peptide-1 (GLP-1) agonist albiglutide reduced the incidence of both type 1 myocardial infarction (HR .73, 95% CI .57–.92) and type 2 myocardial infarctions (HR .65, 95% CI .46–.92) during 1.6-year follow-up (Harmony Outcomes).92 |
| Determining the effectiveness of therapeutic strategies . | Rationale . |
|---|---|
| Antiplatelet therapy | To determine the effectiveness of single or dual antiplatelet therapy in the reduction of future events in patients with type 2 myocardial infarction. |
| Lipid-lowering therapy | The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab reduced the incidence of type 1 (HR .87, 95% CI .77–.99) and type 2 (HR .77, 95% CI .61–.97) myocardial infarction during follow-up (ODYSSEY OUTCOMES trial),29,85,88 demonstrating proof of principle that lipid-lowering therapy may be effective at reducing both type 1 and type 2 myocardial infarction. |
| SGLT2 inhibitors | Empagliflozin was associated with a reduction in future type 1 (RR .79, 95% CI .61–1.04) and type 2 myocardial infarction events (RR .67, 95% CI .41–1.10) at 3.1 years in patients with type 2 diabetes mellitus and cardiovascular disease (EMPA-REG OUTCOME trial).86,89 |
| Coronary revascularization | PCI or CABG compared to medical therapy was associated with a lower mortality in 268 850 patients with type 2 myocardial infarction in a propensity-matched analysis (3.9% vs. 5.5%, OR .70, 95% CI .59–.84, P < .001).90 Prospective studies that minimize the risk of confounding are needed. |
| Blood transfusion in patients with anaemia | The MINT trial demonstrated no difference between a restrictive or liberal transfusion strategy in patients with myocardial infarction (1.15, 95% CI .99–1.34, P = .07). A subgroup analysis suggested a potential signal for harm from a restrictive strategy in type 1 MI (RR 1.32, 95% CI 1.04–1.67) and no difference in type 2 MI (RR 1.05, 95% CI .85–1.29), but this comparison was underpowered.91 |
| GLP-1 agonists | The glucagon-like peptide-1 (GLP-1) agonist albiglutide reduced the incidence of both type 1 myocardial infarction (HR .73, 95% CI .57–.92) and type 2 myocardial infarctions (HR .65, 95% CI .46–.92) during 1.6-year follow-up (Harmony Outcomes).92 |
| Determining the clinical utility of diagnostic imaging . | Rationale . |
|---|---|
| Intravascular imaging (optical coherence tomography or intravascular ultrasound) | To define the frequency of misclassification in those with plaque rupture or erosion with coronary thrombosis. To identify high-risk plaque phenotypes that might increase risk of recurrent myocardial infarction. To systematically evaluate and differentiate subtypes of spontaneous coronary artery dissection. |
| CT coronary angiography | To determine diagnostic performance in type 2 myocardial infarction and ability to discriminate type 1 and type 2 myocardial infarction. To define the prevalence of high-risk plaque phenotypes (calcific plaque, non-calcific plaque, and low-attenuation plaque). |
| Cardiac MRI | To define the frequency of late gadolinium enhancement in type 2 myocardial infarction. To confirm the diagnosis in those with inducible ischaemia and the association with recurrent events. To determine if the prevalence of inducible ischaemia differs in those with type 2 myocardial infarction and acute myocardial injury. |
| Microvascular assessment | To determine the prevalence coronary microvascular disease. |
| Coronary vasospasm | To determine the diagnostic utility of provocation testing for vasomotor dysfunction. |
| Determining the clinical utility of diagnostic imaging . | Rationale . |
|---|---|
| Intravascular imaging (optical coherence tomography or intravascular ultrasound) | To define the frequency of misclassification in those with plaque rupture or erosion with coronary thrombosis. To identify high-risk plaque phenotypes that might increase risk of recurrent myocardial infarction. To systematically evaluate and differentiate subtypes of spontaneous coronary artery dissection. |
| CT coronary angiography | To determine diagnostic performance in type 2 myocardial infarction and ability to discriminate type 1 and type 2 myocardial infarction. To define the prevalence of high-risk plaque phenotypes (calcific plaque, non-calcific plaque, and low-attenuation plaque). |
| Cardiac MRI | To define the frequency of late gadolinium enhancement in type 2 myocardial infarction. To confirm the diagnosis in those with inducible ischaemia and the association with recurrent events. To determine if the prevalence of inducible ischaemia differs in those with type 2 myocardial infarction and acute myocardial injury. |
| Microvascular assessment | To determine the prevalence coronary microvascular disease. |
| Coronary vasospasm | To determine the diagnostic utility of provocation testing for vasomotor dysfunction. |
| Determining the effectiveness of therapeutic strategies . | Rationale . |
|---|---|
| Antiplatelet therapy | To determine the effectiveness of single or dual antiplatelet therapy in the reduction of future events in patients with type 2 myocardial infarction. |
| Lipid-lowering therapy | The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab reduced the incidence of type 1 (HR .87, 95% CI .77–.99) and type 2 (HR .77, 95% CI .61–.97) myocardial infarction during follow-up (ODYSSEY OUTCOMES trial),29,85,88 demonstrating proof of principle that lipid-lowering therapy may be effective at reducing both type 1 and type 2 myocardial infarction. |
| SGLT2 inhibitors | Empagliflozin was associated with a reduction in future type 1 (RR .79, 95% CI .61–1.04) and type 2 myocardial infarction events (RR .67, 95% CI .41–1.10) at 3.1 years in patients with type 2 diabetes mellitus and cardiovascular disease (EMPA-REG OUTCOME trial).86,89 |
| Coronary revascularization | PCI or CABG compared to medical therapy was associated with a lower mortality in 268 850 patients with type 2 myocardial infarction in a propensity-matched analysis (3.9% vs. 5.5%, OR .70, 95% CI .59–.84, P < .001).90 Prospective studies that minimize the risk of confounding are needed. |
| Blood transfusion in patients with anaemia | The MINT trial demonstrated no difference between a restrictive or liberal transfusion strategy in patients with myocardial infarction (1.15, 95% CI .99–1.34, P = .07). A subgroup analysis suggested a potential signal for harm from a restrictive strategy in type 1 MI (RR 1.32, 95% CI 1.04–1.67) and no difference in type 2 MI (RR 1.05, 95% CI .85–1.29), but this comparison was underpowered.91 |
| GLP-1 agonists | The glucagon-like peptide-1 (GLP-1) agonist albiglutide reduced the incidence of both type 1 myocardial infarction (HR .73, 95% CI .57–.92) and type 2 myocardial infarctions (HR .65, 95% CI .46–.92) during 1.6-year follow-up (Harmony Outcomes).92 |
| Determining the effectiveness of therapeutic strategies . | Rationale . |
|---|---|
| Antiplatelet therapy | To determine the effectiveness of single or dual antiplatelet therapy in the reduction of future events in patients with type 2 myocardial infarction. |
| Lipid-lowering therapy | The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab reduced the incidence of type 1 (HR .87, 95% CI .77–.99) and type 2 (HR .77, 95% CI .61–.97) myocardial infarction during follow-up (ODYSSEY OUTCOMES trial),29,85,88 demonstrating proof of principle that lipid-lowering therapy may be effective at reducing both type 1 and type 2 myocardial infarction. |
| SGLT2 inhibitors | Empagliflozin was associated with a reduction in future type 1 (RR .79, 95% CI .61–1.04) and type 2 myocardial infarction events (RR .67, 95% CI .41–1.10) at 3.1 years in patients with type 2 diabetes mellitus and cardiovascular disease (EMPA-REG OUTCOME trial).86,89 |
| Coronary revascularization | PCI or CABG compared to medical therapy was associated with a lower mortality in 268 850 patients with type 2 myocardial infarction in a propensity-matched analysis (3.9% vs. 5.5%, OR .70, 95% CI .59–.84, P < .001).90 Prospective studies that minimize the risk of confounding are needed. |
| Blood transfusion in patients with anaemia | The MINT trial demonstrated no difference between a restrictive or liberal transfusion strategy in patients with myocardial infarction (1.15, 95% CI .99–1.34, P = .07). A subgroup analysis suggested a potential signal for harm from a restrictive strategy in type 1 MI (RR 1.32, 95% CI 1.04–1.67) and no difference in type 2 MI (RR 1.05, 95% CI .85–1.29), but this comparison was underpowered.91 |
| GLP-1 agonists | The glucagon-like peptide-1 (GLP-1) agonist albiglutide reduced the incidence of both type 1 myocardial infarction (HR .73, 95% CI .57–.92) and type 2 myocardial infarctions (HR .65, 95% CI .46–.92) during 1.6-year follow-up (Harmony Outcomes).92 |
Given disease heterogeneity, patient co-morbidity, and frailty, the feasibility of recruiting and randomizing patients with type 2 MI is uncertain. The TARGET-Type 2 feasibility trial (ClinicalTrials.gov NCT:05419583) will randomize patients with type 2 MI 1:1 to usual care, or risk stratification and investigation for coronary artery disease or left ventricular impairment. Where identified, treatment will be recommended on an individual patient basis. Demonstrating that screening, recruitment, and randomization of these patients are feasible is the first step towards the design and delivery of multi-centre trials powered for clinical outcomes.
Summary
Type 2 MI encompasses a variety of underlying pathophysiology. The adoption of this classification in practice has been hampered by this heterogeneity, a lack of objective diagnostic criteria, and evidence from clinical trials to support further investigation and treatment. Whilst this diagnosis is predominantly based on clinical assessment, in some patients this is challenging, and invasive imaging is required. Patients with type 2 MI have poor outcomes, and many have important underlying cardiac disease. Whether further investigation and secondary prevention will benefit all patients with type 2 MI requires investigation in randomized clinical trials.
Supplementary data
Supplementary data are not available at European Heart Journal online.
Declarations
Disclosure of Interest
A.R.C. is supported by a BCIS Advanced Coronary Intervention Fellowship that is in part funded by Abbott Cardiovascular and Edwards Lifesciences. N.L.M. reports receiving honoraria or speaker fees from Abbott Diagnostics, Siemens Healthineers, Roche Diagnostics, LumiraDx, and Psyros Diagnostics within the last 3 years. J.B. has speaker honoraria from Roche, Siemens, Ortho Clinical Diagnostics, Quidel, and Beckmann Coulter, all outside the submitted work.
Data Availability
No data were generated or analysed for this manuscript.
Funding
A.R.C. is supported by a British Cardiovascular Interventional Society (BCIS) Advanced Coronary Interventional Fellowship. N.L.M. is supported by the British Heart Foundation through a Chair Award (CH/F/21/90010), a Programme Grant (RG/20/10/34966), and a Research Excellence Award (RE/24/130012) from the British Heart Foundation.



