-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, Focus on amyloidosis, peripartum cardiomyopathy, and heart failure prediction by artificial intelligence applied to ECG, European Heart Journal, Volume 46, Issue 11, 14 March 2025, Pages 987–990, https://doi.org/10.1093/eurheartj/ehaf084
Close - Share Icon Share
 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
This Focus Issue on heart failure and cardiomyopathies contains the State of the Art Review entitled ‘Transthyretin amyloid cardiomyopathy: a paradigm for advancing precision medicine’ by Esther Gonzalez-Lopez from the Hospital Universitario Puerta de Hierro Majadahonda in Madrid, Spain, and colleagues.1 The authors point out that development of specific therapies addressing the mechanisms of the underlying diseases constitutes the basis of precision medicine. Transthyretin cardiac amyloidosis (ATTR-CM) is an exemplar of a precise therapeutic approach in the field of heart failure (HF) and cardiomyopathies.2–5 A better understanding of the underlying pathophysiology, more precise data on its epidemiology, and advances in imaging techniques that allow non-invasive diagnosis have fostered the development of new and very effective specific therapies for ATTR-CM. Therapeutic advances have revolutionized the field, transforming a rare, devastating, and untreatable disease into a more common disease with several therapeutic alternatives available. Three main types of therapies (stabilizers, suppressors, and degraders) that act at different points of the amyloidogenic cascade have been developed or are currently under investigation. In this review, the key advances in pathophysiology and epidemiology that have occurred in the last decades along with the different therapeutic alternatives available or under development for ATTR-CM are described, illustrating the role of precision medicine applied to cardiovascular disorders. Pending questions that would need to be answered in upcoming years are also reviewed.
In a Viewpoint article entitled ‘The year in cardiovascular medicine 2024: the top 10 papers in heart failure’, EHJ Editors Johann Bauersachs, Shelley Zieroth, and Rudolf de Boer have compiled 10 impactful papers published in 2024 on heart failure (HF).6 This is the first contribution this year to this successful series of articles.
Peripartum cardiomyopathy (PPCM) remains a serious threat to maternal health around the world.7–11 While bromocriptine, in addition to standard treatment for HF, presents a promising pathophysiology-based disease-specific treatment option in PPCM, the evidence regarding its efficacy remains limited. In a Fast Track Clinical Research article entitled ‘Bromocriptine treatment and outcomes in peripartum cardiomyopathy: the EORP PPCM registry’, Peter van der Meer from the University of Groningen in the Netherlands, and colleagues aimed to determine whether bromocriptine treatment is associated with improved maternal outcomes in PPCM.12 Peripartum cardiomyopathy patients from the EORP PPCM registry with available follow-up were included. The main exposure of this exploratory non-randomized analysis was bromocriptine treatment, and the main outcome was a composite endpoint of maternal outcome (death or hospital readmission within the first 6 months after diagnosis, or persistent severe left ventricular dysfunction [left ventricular ejection fraction {LVEF} < 35%] at 6-month follow-up). Inverse probability weighting was used to minimize the effects of confounding by indication. Multiple imputation was used to account for the missing data. Among the 552 patients with PPCM, 85 were treated with bromocriptine (15%). The primary endpoint was available in 89% and occurred in 22% of patients treated with bromocriptine in addition to standard of care and in 33% of patients treated only with standard of care (P = .044). In complete case analysis, bromocriptine treatment was associated with reduced adverse maternal outcome (odds ratio [OR] 0.29, P = .021). This association remained after applying multiple imputation and methods to correct for confounding by indication (inverse probability weighted model on imputed data: OR 0.47, P < .001).
The authors conclude that among women with PPCM, bromocriptine treatment in addition to standard of care is associated with better maternal outcomes after 6 months. The contribution is accompanied by an Editorial by Uri Elkayam from the Los Angeles General Hospital and the Foundation of Heart Failure Education in Los Angeles, CA, USA.13 Elkayam highlights that although the results of this study could have implications on the management of PPCM all over the world, because of the important differences in the outcome of patients with PPCM in different geographical regions, investigators and funding institutions in other countries should be encouraged to perform similar, well-designed studies to evaluate the therapeutic potential of bromocriptine in different PPCM populations.
The risk of HF progression or mortality in patients with PPCM during subsequent pregnancies (SSPs) is a significant concern for patients, their families, and healthcare providers. However, there are limited contemporary, prospective data on SSP outcomes in PPCM patients from diverse ethnic and sociodemographic groups. In a Clinical Research article entitled ‘Pregnancies in women after peripartum cardiomyopathy: the global ESC EORP PPCM registry’, Karen Sliwa from the University of Cape Town in South Africa and colleagues aimed to assess maternal and neonatal outcomes in PPCM patients undergoing SSPs.14 From 332 patients with PPCM, there were 98 SSPs among 73 women. Of these, 25 (26%) SSPs ended prematurely due to therapeutic termination, miscarriage, and stillbirth. Median follow-up from the end of the SSP was 198 days. LVEF was persistently reduced to <50% prior to the SSP in 26% of patients. Clinical worsening (composite of all-cause death, cardiovascular rehospitalization, or decline in LVEF ≥10% [percentage points] and to <50%) occurred in 20% SSPs, with 2% all-cause maternal mortality. Signs/symptoms of HF and worsening of New York Heart Association class occurred in 26% and 22% of SSPs, respectively. At follow-up, mean LVEF was 50% and in 69% of SSPs LVEF was ≥50%. African women had similar outcomes to the other ethnic groups. Pre-term delivery occurred in 24% of SSPs, 20% of babies were of low birth weight, and there was 3% all-cause neonatal mortality. Compared with women with SSP baseline LVEF <50%, fewer women with LVEF ≥50% were on HF pharmacotherapies prior to the SSP and, in this group, a significant decline in LVEF was observed (Figure 1).
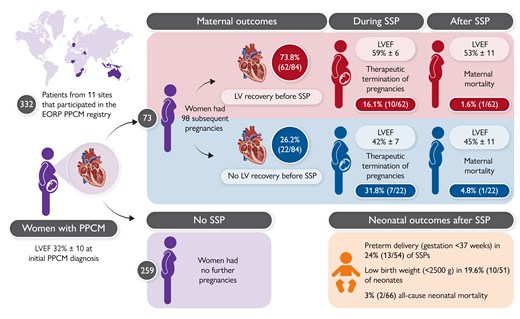
Outcome of pregnancies in women with a previously diagnosed Peripartum Cardiomyopathy. EORP, EuroObservational Research Programme; LV, left ventricular; LVEF, LV ejection fraction; PPCM, peri-partum cardiomyopathy; SSP, subsequent pregnancy.14
Sliwa and colleagues conclude that maternal morbidity and mortality rates are lower than anticipated. Baseline LVEF <50% is not associated with an increased frequency of adverse maternal outcomes, and no further decline in LVEF is observed in this group. In contrast, women with SSPs and a baseline LVEF ≥50% experience a decline in LVEF, potentially attributable to reduced use of HF pharmacotherapy during pregnancy and the post-partum period. This manuscript is accompanied by an Editorial by Olayinka Agboola and Garima Sharma from the Inova Health System in Falls Church, VA, USA.15 The authors conclude that the manuscript provides additional insights into a challenging patient population that deserves nuanced care and thoughtful consideration. Studies like these, even though descriptive, provide an avenue for more careful delivery planning considerations in this high-risk population.
Current HF risk stratification strategies require comprehensive clinical evaluation.16–21 In a Clinical Research article entitled ‘Heart failure risk stratification using artificial intelligence applied to electrocardiogram images: a multinational study’, Lovedeep Dhingra from the Yale School of Medicine in New Haven, CT, USA, and colleagues examined artificial intelligence (AI) applied to electrocardiogram (ECG) images as a strategy to predict HF risk.22 Across multinational cohorts in the Yale New Haven Health System (YNHHS), UK Biobank (UKB), and Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), individuals without baseline HF were followed for the first HF hospitalization. An AI-ECG model that defines cross-sectional left ventricular systolic dysfunction from 12-lead ECG images was used, and its association with incident HF was evaluated. Among >231 000 YNHHS patients, ∼4500 had primary HF hospitalizations over 4.5 years. Similar prevalences were observed in the other cohorts. A positive AI-ECG screen portended a 4- to 24-fold higher risk of new-onset HF. The association was consistent after accounting for comorbidities and the competing risk of death. Higher probabilities were associated with progressively higher HF risk. Model discrimination was 0.718 in YNHHS, 0.769 in UKB, and 0.810 in ELSA-Brasil.
The authors conclude that an AI model applied to a single ECG image defines the risk of future HF, representing a digital biomarker for stratifying HF risk. The contribution is accompanied by an Editorial by Charalambos Antoniades and Kenneth Chan from the University of Oxford in the UK.23 The authors note that a key feature of the AI-ECG model described by Dhingra et al. is the use of ECG images (rather than raw digital time/voltage ECG data) as an input parameter. In the model development, images of ECG waveforms in the standard clinical format were resized and transformed into >10 million trainable parameters as input to the convolutional neural network. This approach eliminates the constraints of extracting raw ECG voltage data which is vendor specific and not always accessible. They conclude that the ability to predict future HF from ECG waveforms, as presented by Dhingra et al., is a major step towards implementing low-cost population screening in search of the hidden signs of HF.
Wild-type transthyretin amyloid cardiomyopathy (ATTRwt-CM) is increasingly diagnosed in patients ≥80 years old (octogenarians), although being under-represented in randomized clinical trials. Specific data on the natural course and outcome under tafamidis treatment in octogenarians are therefore scarce. In a Clinical Research article entitled ‘Tafamidis in octogenarians with wild-type transthyretin cardiac amyloidosis: an international cohort study’, Philippe Debonnaire from the AZ Sint-Jan Brugge-Oostende AVRinggold in Bruges, Belgium, and colleagues studied the impact of tafamidis treatment on mortality in real-world ATTRwt-CM octogenarians.24 In an international, multicentre cohort study, 710 consecutive ATTRwt-CM patients with a mean follow-up of 2.2 ± 1.8 years were studied for the endpoint of all-cause mortality. The cohort included 58% of octogenarians (85 ± 4 years old, 74% male). Before tafamidis availability, the natural course in octogenarians versus non-octogenarians was poor, with 16% one year and 71% five year mortality vs 8% and 47%, respectively (P < .001). Since tafamidis availability, 70% of octogenarians were initiated on tafamidis, vs 84% of non-octogenarians (P < .001). In octogenarians, tafamidis treatment was associated with lower mortality after propensity score matching on baseline variables, including age, stage, and NYHA class (hazard ratio [HR] 0.57, P = .053). Neither age at diagnosis nor age at treatment initiation interacted with tafamidis mortality benefit (Figure 2).
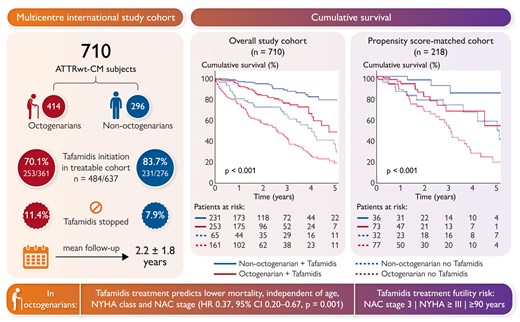
Treatable cohort: since tafamidis availability for ATTRwt-CM indication. ATTRwt-CM, wild-type transthyretin cardiomyopathy; CI, confidence interval; HR, hazard ratio; NAC, National Amyloidosis Centre prognostic stage; NYHA, New York Heart Association.24
The authors conclude that tafamidis treatment in the real world improves survival without age affecting treatment efficacy, although mortality remains considerable in octogenarians. The manuscript is accompanied by an Editorial by Marianna Fontana from the University College London in the UK, together with Carlo Fumagalli and Francesco Cappelli from the Careggi University Hospital in Florence, Italy.25 The authors conclude that while the study of Debonnaire et al. provides valuable insights into the potential benefits of tafamidis in octogenarians with ATTR-CM, it also highlights the necessity of looking beyond age as the sole factor in treatment decisions. Validation of geriatric assessment tools and integration of a comprehensive assessment of geriatric syndromes into clinical practice will be essential for optimizing care in elderly patients with ATTRwt-CM.
In a Rapid Communications article entitled ‘Anaemia, erythrocytosis, and empagliflozin in heart failure with preserved ejection fraction: the EMPEROR-Preserved trial’, João Pedro Ferreira from the Faculty of Medicine of the University of Porto in Portugal and colleagues remind us that anaemia worsens HF symptoms and has been associated with poor cardiovascular outcomes.26 Sodium–glucose co-transporter 2 inhibitors (SGLT2is) increase haemoglobin (and haematocrit), often leading to the correction of anaemia and, in some patients, SGLT2i use may lead to erythrocytosis. While the association of anaemia with poor outcomes has been documented in HF, the association of erythrocytosis with outcomes in HF is less well established. In the EMPEROR trial at baseline, 2.1% of patients had erythrocytosis. In the placebo group, the presence of anaemia at baseline was independently associated with an increased risk of occurrence of all studied outcomes compared with patients without anaemia. The HR for the primary outcome was 1.66 (P < .001). The presence of erythrocytosis at baseline was not associated with any of the studied outcomes compared with patients without erythrocytosis and was not associated with atherothrombotic events. Empagliflozin (vs placebo) reduced the odds of experiencing anaemia by 40% at week 52: OR 0.58 (P < .001). On the other hand, empagliflozin (vs placebo) increased the odds of experiencing erythrocytosis (OR 3.29 at week 52; P < .001). An increase in haemoglobin levels was associated with higher odds of meaningful (≥5 points) Kansas City Cardiomyopathy Questionnaire clinical summary score improvement (and lower odds of deterioration) among patients randomized to empagliflozin but not among those randomized to placebo.
The authors conclude that the present study adds novel and clinically relevant information: (i) the treatment effect of empagliflozin is not influenced by anaemia status and empagliflozin leads to an important reduction of anaemia throughout the follow-up; (ii) empagliflozin increases the proportion of patients with erythrocytosis throughout the follow-up, but erythrocytosis is not associated with atherothrombotic events.
The editors hope that this issue of the European Heart Journal will be of interest to its readers.
Dr. Crea reports speaker fees from Abbott, Amgen, Astra Zeneca, BMS, Chiesi, Daiichi Sankyo, Menarini outside the submitted work.
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.




