-
PDF
- Split View
-
Views
-
Cite
Cite
Mandeep R Mehra, Francesco Castagna, Javed Butler, The transformative potential of left ventricular assist devices in advanced heart failure: no more a therapeutic orphan, European Heart Journal, Volume 45, Issue 8, 21 February 2024, Pages 626–628, https://doi.org/10.1093/eurheartj/ehad555
Close - Share Icon Share
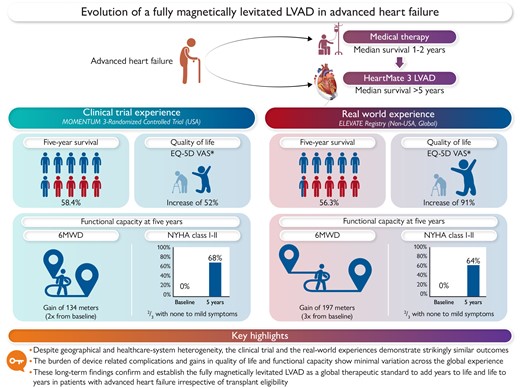
A comparison of ‘within trial’ (MOMENTUM 3) and ‘real-world’ (ELEVATE) long-term (5-year) outcomes with a fully magnetically levitated left ventricular assist device (LVAD). These data show marked similarity across the domains of survival, quality of life, and functional capacity irrespective of transplant eligibility, geographic location, ethnic population differences, and diverse healthcare systems. (This figure was created with BioRender.com).
This editorial refers to ‘Fully magnetically centrifugal left ventricular assist device and long-term outcomes: the ELEVATE registry’, by J.D. Schmitto et al., https://doi.org/10.1093/eurheartj/ehad658.
Even as we applaud progress in medical therapy for the millions of patients with chronic heart failure, 10%–15% of such patients transition into advanced heart failure, a clinical state characterized by intolerance to neurohormonal disease-modifying drug therapy and development of cardio-renal perturbations accompanied by a high symptom burden, worsening exercise capacity, and recurrent hospitalization requiring frequent intensified care.1 Until the introduction of durable mechanical circulatory support, such patients were ‘therapeutic orphans’ with limited options to relieve suffering and prolong life. A small fraction of patients without significant comorbidity burden and with well-established support systems may qualify for heart transplantation, but the majority often require chronic inotropic support or disease palliation.1 Implantable left ventricular assist devices (LVADs) offer a paradigm of an ‘off the shelf’ option to support the failing heart as a bridge to facilitate successful transplantation during the long wait for scarce donor organs, or to provide a long-term therapeutic alternative to those patients unable to qualify for transplantation.2
Early mechanical circulatory support pumps were introduced under the presumption that they needed to replicate the physiology of a valve-supported pulsatile chamber. This required development of bulky devices with valved conduits and displacement chambers, each of which was prone to malfunction. Thus, the observed benefits were short lasting and often accompanied by morbid complications of thrombo-embolism, bleeding, infection, and pump failure, with death resulting from multi-organ system failure.2 After the turn of the millennium, a major development in LVAD engineering ensued, with introduction of continuous-flow non-pulsatile smaller pumps which could prolong life well beyond a few months and instead provide several years of function-enhancing support as a consequence of greater device durability.2 It was this technological advance that altered the construct of LVADs from a short-term bridge or rescue therapy to one of meaningful life prolongation. This allowed emergence of the notion of lifetime support or destination therapy even in patients with advanced heart failure ineligible for transplantation.
The improved outcomes with continuous-flow pumps were exemplified by an axial flow device [the HeartMate II LVAD, Abbott (USA)] or a centrifugal flow device [Heartware LVAD, Medtronic (Ireland)], both of which prolonged survival but at a devastating cost of disabling haemocompatibility-related adverse effects which included stroke (more commonly with the Heartware LVAD) and pump thrombosis requiring repeat surgical pump replacement (more common with the Heart Mate II LVAD), as well as a high burden of gastrointestinal bleeding due to their effects on circulating blood elements.2,3 As a result of these adverse effects, the burden of hospitalization was substantial, quality of life gain was reduced, and challenges of cost-effectiveness emerged, which in concert slowed widespread global adoption.
In 2015, the first clinical results with a novel fully magnetically levitated rotor pump, the HeartMate 3 LVAD were reported.4 This LVAD was uniquely engineered to overcome the problem of de novo pump thrombosis which required pump removal or replacement in most instances. The beneficial engineering attributes of this LVAD included wide blood flow pathways, a frictionless rotor, and an asynchronous device pulse (created to ‘wash out’ the pump and avoid thrombosis). Whether these attributes translated into clinical benefit over and above the prevailing technology was promising but clinically unproven. In 2019, The Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) randomized clinical trial of 1028 patients reported its final 2-year outcomes and concluded the superiority of the HeartMate 3 LVAD compared with the HeartMate II pump on haemocompatibility-related adverse events, with demonstration of near elimination of pump thrombosis, a marked reduction in stroke rates, and a modest decrease in gastrointestinal bleeding.5 However, in the 2-year time frame, there was no clear survival advantage between these two pumps. Until then, the HeartMate II and HVAD pumps had been shown to confer modest gains in survival, and whether the clinical superiority of the HeartMate 3 LVAD prolonged late survival beyond that of these prior generational pumps remained uncertain.2
In a 5-year analysis of the MOMENTUM 3 trial, reported in 2022, it became clear that the marked reduction in haemocompatibility-related adverse events also translated into a survival advantage with the HeartMate 3 LVAD, with an increase in median survival beyond 5 years.6 Simultaneously, it also became known that implantation of the HeartMate 3 LVAD not only prolonged survival substantially but reduced the burden of complication-related hospitalizations, provided a marked improvement in quality of life, and increased functional capacity irrespective of transplantation eligibility.5,6,7 All these attributes in healthcare resource utilization were noted to confer greater cost-effectiveness across geographically diverse regions.7,8 Whether these controlled results from within a clinical trial could be replicated in the real world was less certain, since some argued that <50% of patients undergoing HeartMate 3 implantation in the real-world mimicked trial-like patients.9
In this issue of the European Heart Journal, Schmitto and colleagues10 present the much-anticipated findings of the 5-year outcomes of ELEVATE, a real-world prospective registry of implantation of the HeartMate 3 LVAD in 540 patients across a broad global population attained from 26 centres and 11 countries which included the UK, the European Union (7 countries), Israel, Kazakhstan, and Singapore. These investigators report a striking similarity to the outcomes noted within the MOMENTUM 3 trial, with a median survival that exceeds 5 years, a low rate of haemocompatibility-related adverse events, and gains in quality of life and functional capacity that are attained early and preserved to the 5-year time frame without diminution (Graphical Abstract). These external validation findings, which enrolled patients at a time between 2015 and 2017 while the MOMENTUM 3 trial was still underway, are important since they demonstrate that these benefits of the HeartMate 3 LVAD are independent of geographic region, patient phenotype (bridge or destination therapy), or ethnicity, and show validity across diverse healthcare systems and care delivery models. Despite challenges of a registry which do not allow for event adjudication and are prone to event classification errors (as an example, the registry noted an unusually lower than previously described rate of residual heart failure events), these findings should provide assurance to clinicians on the expected magnitude of benefit of the HeartMate 3 LVAD in advanced heart failure. Although most patients in this registry were allocated to a bridge to transplant strategy, the 5-year rates of transplantation were <1 in 5, indicating the lack of donor organ availability. These low transplant rates, however, suggest that ongoing support with the HeartMate 3 LVAD continues to provide clinically meaningful gains in quality and duration of life irrespective of the opportunity for transplantation.
We believe that we have now ushered in a new era in therapeutic opportunity for patients with advanced heart failure, the benefits of which cannot be ignored. Challenges remain on perceptions of LVAD therapy by the clinical community at large who still view this as rescue therapy for actively declining heart failure patients who are often unclear on the benefits of life prolongation compared with the base-case of use of inotropic therapy and tend to overestimate the effectiveness of medical therapy in severe heart failure. Clinicians, patients, and families often perceive the journey with an LVAD as demanding, complex, and resource intensive. Most clinicians are unable to articulate the benefits of LVAD therapy to patients and their care providers, and wait for evaluation by an advanced heart failure centre.11 In doing so, patients reach the doorstep of an advanced heart failure programme in extreme stages of illness with worsened end-organ morbidity, and have a need to establish trust with new care teams at a time when they are often under psychological distress while they confront difficult life choices. In this regard, we have attempted to create tools for use by clinicians to ensure effective early communication with patients considering LVAD therapy, provide personalized risk scores for prediction of outcomes to prepare patients and their families for what their individual journey may anticipate, and to appreciate not only life-changing benefits but also trade-offs that are likely to be encountered.11,12
As with any therapy, there is always an opportunity for incremental enhancement in outcomes. We now know that patients who are ineligible for transplantation can achieve outcomes with LVAD therapy that may be indistinguishable from those gains anticipated with organ replacement therapy. Yet, we must confront challenges of residual heart failure and infection due to the necessity of a driveline required to power the pump using external energy sources.13,14 Fortunately, engineering continues to advance this field, and we shall see advent of novel devices that provide more physiological and synchronized pulsatile flow (rather than the unnatural trans-apical to aortic flow with current LVADs).15 While today’s devices are forgiving, they are not forgettable. The next paradigm shift will be defined with more natural and pulsatile flow LVADs that are physiologically responsive (smart pumps), forgiving in adverse events (more biocompatible), and forgettable (without onerous external peripheral components). Even as we look towards a more evolved future with support technology, we must not ignore the vividly present opportunity to relieve the ‘therapeutic orphan’ status of advanced heart failure and reduce such suffering.
Declarations
Disclosure of Interest
M.R.M. reports payment made to his institution from Abbott for consulting; consulting fees from Mesoblast, Janssen, Moderna, Paragonix, and Baim Institute for Clinical Research; and is an Advisory Board member for Transmedics, NuPulseCV, Leviticus, and FineHeart. J.B. reports serving as a consultant to Abbott, American Regent, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardiac Dimension, Cardior, CVRx, Cytokinetics, Edwards, Element Science, Innolife, Impulse Dynamics, Imbria, Inventiva, Lexicon, Lilly, LivaNova, Janssen, Medtronics, Merck, Occlutech, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Pharmain, Roche, Sequana, SQ Innovation, and Vifor. F.C. has no relevant disclosures.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.



