-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, Beyond traditional cardiovascular risk factors: exploring the hidden side of the moon, European Heart Journal, Volume 45, Issue 6, 7 February 2024, Pages 407–410, https://doi.org/10.1093/eurheartj/ehae055
Close - Share Icon Share
 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
This Focus Issue on epidemiology, prevention, and healthcare policies contains the State of the Art Review article ‘Exposome in ischaemic heart disease: beyond traditional risk factors’ by Rocco Antonio Montone from the Policlinico Universitario Agostino Gemelli in Rome, Italy, and colleagues.1 The authors point out that ischaemic heart disease represents the leading cause of morbidity and mortality, typically induced by the detrimental effects of risk factors on the cardiovascular system. Despite the fact that preventive interventions based on tackling conventional risk factors have helped to reduce the incidence of ischaemic heart disease, it remains a major cause of death worldwide.2–7 Thus, attention is now shifting to non-traditional risk factors in the built, natural, and social environments that collectively contribute substantially to the burden of disease and perpetuate residual risk.8,9 Of importance, these complex factors interact non-linearly and in unpredictable ways to often enhance the detrimental effects attributable to a single factor or collection of these factors. For this reason, a new paradigm called the exposome has been recently introduced by epidemiologists in order to define the totality of exposure to these new risk factors. The purpose of this review is to outline how these emerging risk factors may interact and contribute to the occurrence of ischaemic heart disease, with particular attention on the impact of long-term exposure to different environmental pollutants, socioeconomic and psychological factors, along with infectious diseases such as influenza and COVID-19. Moreover, potential mitigation strategies are discussed for both individuals and communities.
Hypertriglyceridaemia is an important risk factor for cardiovascular morbidity and mortality.10 In a Viewpoint article entitled ‘Icosapent ethyl for hypertriglyceridaemia and atherosclerosis: greater RESPECT for increased therapeutic use’, Prakriti Gaba from the Harvard Medical School in Boston, MA, USA, and colleagues note that with a prevalence of >10% in the adult population, hypertriglyceridaemia remains a significant burden in the cardiovascular community.11 A variety of pharmacotherapeutic agents have been developed to target elevated triglyceride levels and to potentially mitigate the adverse cardiovascular sequelae that often accompany them. These agents include focused triglyceride-lowering interventions such as high-dose omega-3 fatty acids, fibrates, and niacin, as well as LDL-cholesterol-lowering medications, like statins and proprotein convertase subtilisin/kexin 9 inhibitors. Omega-3 fatty acids with highly purified pharmaceutical grade eicosapentaenoic acid (EPA), or icosapent ethyl (IPE), have been of particular interest, given their ability to reduce adverse cardiovascular events. The authors highlight that we now have evidence from an amalgamation of sources, such as randomized clinical trials, prospective imaging studies, and basic science experiments, that support the use of IPE in the treatment of patients with even mild hypertriglyceridaemia and elevated cardiovascular risk. The US Food & Drug Administration has approved IPE as an adjunct to maximally tolerated statin therapy for mitigating cardiovascular risk in patients with triglyceride levels ≥150 mg and either established cardiovascular disease or diabetes plus additional cardiovascular risk factors. The risks of the medication are few and the benefits substantial. As such, the main therapeutic focus moving forward should be enhanced implementation of this therapy in appropriate patients.
Low birth weight is a common pregnancy complication, which has been associated with higher risk of cardiometabolic disease in later life. In a Fast Track Clinical Research article entitled ‘Birth weight influences cardiac structure, function, and disease risk: evidence of a causal association’, Maddalena Ardissino from Imperial College London in the UK, and colleagues indicate that prior Mendelian randomization (MR) studies exploring this question do not distinguish the mechanistic contributions of variants that directly influence birth weight through the foetal genome (direct foetal effects) vs. variants influencing birth weight indirectly by causing an adverse intrauterine environment (indirect maternal effects).12 In this study, MR was used to assess whether birth weight, independent of intrauterine influences, is associated with cardiovascular disease risk and measures of adverse cardiac structure and function. Uncorrelated (r2 < .001), genome-wide significant (P < 5 × 10−8) single nucleotide polymorphisms were extracted from the summary statistics of genome-wide association studies for birth weight overall, and after isolating direct foetal effects only. Inverse-variance weighted MR was utilized for analyses on outcomes of atrial fibrillation, coronary artery disease, heart failure, ischaemic stroke, and 16 measures of cardiac structure and function. Multiple comparisons were accounted for by Benjamini–Hochberg correction. Lower genetically predicted birth weight, isolating direct foetal effects only, was associated with an increased risk of coronary artery disease (odds ratio 1.21, 95% confidence interval 1.06–1.37; P = .031), smaller chamber volumes, and lower stroke volume, but higher contractility (Figure 1).
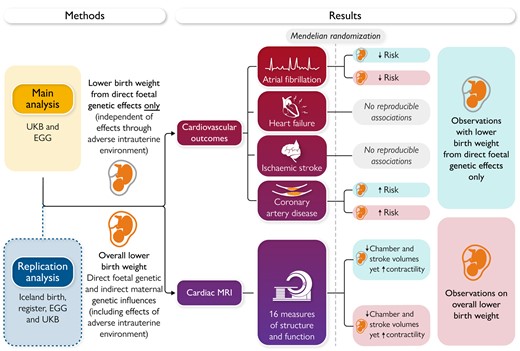
Mendelian randomization study evaluating the causal relevance of birth weight on cardiovascular structure, function, and disease risk. UKB, UK Biobank; EGG, Early Growth Genetics Consortium; MRI, magnetic resonance imaging.12
The authors conclude that the results of this study support a causal role for low birth weight in cardiovascular disease, even after accounting for the influence of the intrauterine environment. This suggests that individuals with a low birth weight may benefit from early targeted cardiovascular disease prevention strategies, independent of whether this was linked to an adverse intrauterine environment during gestation. The contribution is accompanied by an Editorial by Maryam Kavousi from Erasmus University Medical Center in Rotterdam and Harold Snieder from the University of Groningen in the Netherlands.13 The authors note that at least part of the association between low birth weight and coronary heart disease was mediated by cardiovascular risk factors including systolic blood pressure and type 2 diabetes. This calls for life course approaches to identify and treat early indices of cardiovascular and metabolic risk. While the cardiovascular disease causation pathways are complex, there is a range of (i) common risk factors that are modifiable, such as smoking, unhealthy diets, physical inactivity, and harmful use of alcohol; and (ii) intermediate risk factors which provide important targets for early intervention in clinical medicine in terms of cardiovascular risk assessment and therapy, such as raised blood sugar or blood pressure, unfavourable blood lipid profile, and overweight/obesity. Results of the study by Ardissino and colleagues highlight the preventive potential for more intensive early screening and control for high blood pressure and dysglycaemia and routine surveillance of cardiovascular structure and function among individuals with a history of low birth weight.
Physical inactivity, sedentary behaviour (SB), and inadequate sleep are key behavioural risk factors of cardiometabolic diseases.14–18 Each behaviour is mainly considered in isolation, despite clear behavioural and biological interdependencies. In a Clinical Research article entitled ‘Device-measured physical activity and cardiometabolic health: the Prospective Physical Activity, Sitting, and Sleep (ProPASS) consortium’, Joanna Blodgett from the University College London in the UK, and colleagues investigate associations of device-measured physical activity with adiposity and cardiometabolic biomarkers.19 Cross-sectional data from six studies (n = 15 253 participants; five countries) from the Prospective Physical Activity, Sitting, and Sleep consortium were analysed. Device-measured time spent in sleep, SB, standing, light-intensity physical activity (LIPA), and moderate-vigorous physical activity (MVPA) was assessed. Outcomes included body mass index (BMI), waist circumference, HDL-cholesterol, total:HDL-cholesterol ratio, triglycerides, and glycated haemoglobin (HbA1c). Compositional linear regression examined associations between physical activity and outcomes, including modelling time reallocation between behaviours. The average daily composition of the sample (mean age: 54 years; 55% female) was 7.7 h sleeping, 10.4 h sedentary, 3.1 h standing, 1.5 h LIPA, and 1.3 h MVPA. Reallocating time from SB, standing, LIPA, or sleep into MVPA resulted in better scores across all outcomes. For example, replacing 30 min of SB, sleep, standing, or LIPA with MVPA was associated with −0.63, −0.43, −0.40, and −0.15 kg/m2 lower BMI, respectively. Greater relative standing time was beneficial, whereas sleep had a detrimental association when replacing LIPA/MVPA and a positive association when replacing SB. The minimal displacement of any behaviour into MVPA for improved cardiometabolic health ranged from 3.8 (HbA1c) to 12.7 (triglycerides) min/day.
The authors conclude that compositional data analyses reveal a distinct hierarchy of behaviours. MVPA demonstrates the strongest, most time-efficient protective associations with cardiometabolic outcomes. Theoretical benefits from reallocating SB into sleep, standing, or LIPA requires substantial changes in daily activity. This manuscript is accompanied by an Editorial by Genevieve Healy from the The University of Queensland in Australia.20 The authors note that the study by Blodgett and colleagues highlights the interplay between movement behaviours across the 24-h day and adds to the evidence base on the importance of MVPA participation. It also reinforces the detrimental associations of high levels of SB with indicators of cardiometabolic health, and suggests that switching at least some of this SB to standing may be of benefit—particularly when MVPA may not be feasible. In practical terms, these findings should be used to discuss movement behaviours across the 24-h day to help ensure that an appropriate balance is achieved for good heart health.
Recreational cannabis use has been linked with cardiovascular side effects,21 necessitating investigations concerning the safety of prescribed medical cannabis. Indeed, a rising number of countries allow physicians to treat chronic pain with medical cannabis. In a Clinical Research article entitled ‘Cannabis for chronic pain: cardiovascular safety in a nationwide Danish study’, Anders Holt from the Copenhagen University Hospital—Herlev and Gentofte in Denmark, and colleagues22 used nationwide Danish registers to identify patients with chronic pain initiating first-time treatment with medical cannabis during 2018–21 who were matched 1:5 to corresponding control patients on age, sex, chronic pain diagnosis, and concomitant use of other pain medication. The absolute risks of first-time arrhythmia (atrial fibrillation/flutter, conduction disorders, paroxysmal tachycardias, and ventricular arrhythmias) and acute coronary syndrome were reported comparing medical cannabis use with no use. Among 1.88 million patients with chronic pain, 5391 patients claimed a prescription of medical cannabis (63% women, median age: 59 years). Medical cannabis use was associated with a significantly higher risk of new-onset arrhythmia (risk ratio 2.07). No significant association was found for acute coronary syndrome (Figure 2).
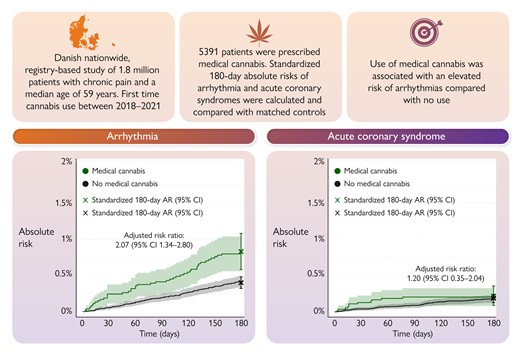
Medical cannabis and cardiovascular risk. A graphical representation of the main findings showing the risk of new-onset arrhythmia and acute coronary syndrome in patients with chronic pain according to the use of medical cannabis. AR, absolute risk; CI, confidence interval.22
Holt et al. conclude that in patients with chronic pain, the use of prescribed medical cannabis is associated with an elevated risk of new-onset arrhythmia compared with no use—most pronounced in the 180 days following the initiation of treatment. The contribution is accompanied by an Editorial by Robert L. Page II from the University of Colorado in Aurora, CO, USA.23 Page highlights that the investigators of this robust analysis should be commended as their findings add to the growing evidence that as with all psychotropic agents, adverse effects, particularly cardiovascular, can occur with cannabis, regardless of its name.
The issue is also complemented by a Discussion Forum contribution. In a commentary entitled ‘Pre-race aspirin to attenuate the risk for marathon-related cardiac arrest: deconstructing the legacy of Pheidippides’, Arthur J. Siegel from the Massachusetts General Hospital in Boston, MA, USA comments on the recent publication ‘Lifelong endurance exercise and its relation with coronary atherosclerosis’ by Ruben De Bosscher from the University Hospitals Leuven in Belgium.24,25
The editors hope that this issue of the European Heart Journal will be of interest to its readers.
Dr. Crea reports speaker fees from Abbott, Amgen, Astra Zeneca, BMS, Chiesi, Daiichi Sankyo, Menarini outside the submitted work.
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.




