-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, The dark side of arrhythmia treatment: iatrogenic tricuspid regurgitation and drug toxicity, European Heart Journal, Volume 45, Issue 5, 1 February 2024, Pages 317–320, https://doi.org/10.1093/eurheartj/ehae026
Close - Share Icon Share
 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
This Issue opens with the State of the Art Review article entitled ‘Artificial intelligence: revolutionizing cardiology with large language models’ by Machteld Boonstra from the University of Amsterdam in the Netherlands, and colleagues.1 Artificial intelligence is playing a growing role in cardiovascular medicine.2–8 The authors note that natural language processing techniques are having an increasing impact on clinical care from the patient, clinician, administrator, and research perspective. Among others are automated generation of clinical notes and discharge letters, medical term coding for billing, medical chatbots for both patients and clinicians, data enrichment in the identification of disease symptoms or diagnosis, cohort selection for clinical trials, and auditing purposes. In the review, an overview of the history of natural language processing techniques developed, with a brief technical background, is presented. Subsequently, the review discusses implementation strategies of natural language processing tools, thereby specifically focusing on large language models, and concludes with future opportunities in the application of such techniques in the field of cardiology.
The Issue continues with a focus on arrhythmias. In a State of the Art Review article entitled ‘Tricuspid valve disease and cardiac implantable electronic devices’, Martin Andreas from the Medical University of Vienna in Austria, and colleagues note that the role of cardiac implantable electronic device (CIED)-related tricuspid regurgitation (TR) is increasingly recognized as an independent clinical entity.9 They also indicate that novel surgical and interventional tricuspid valve treatment options are increasingly applied to patients suffering from TR associated with or related to CIEDs. This multidisciplinary review article developed with electrophysiologists, interventional cardiologists, imaging specialists, and cardiac surgeons aims to give an overview of the mechanisms of disease and diagnostics, and proposes treatment algorithms for patients suffering from TR associated with CIED lead(s) or leadless pacemakers.
While a strong body of knowledge has been gathered in the past few years on risk factors and management of atrial fibrillation (AF),10–17 data on new-onset AF (NOAF) in patients with chronic coronary syndrome (CCS) are scarce. In a Fast Track Clinical Research article entitled ‘New-onset atrial fibrillation and chronic coronary syndrome in the CLARIFY registry’, Alexandre Gautier from the Hôpital Bichat, Assistance Publique-Hôpitaux de Paris in France, and colleagues aim to describe the incidence, predictors, and impact on cardiovascular (CV) outcomes of NOAF in CCS patients.18 Data from the international (45 countries) CLARIFY registry (prospeCtive observational LongitudinAl RegIstry oF patients with stable coronary arterY disease) were used. Among 29 001 CCS outpatients without previously reported AF at baseline, patients with at least one episode of AF/flutter diagnosed during 5-year follow-up were compared with patients in sinus rhythm throughout the study. The incidence rate of NOAF was 1.12 per 100 patient-years (cumulative incidence at 5 years: 5.0%). Independent predictors of NOAF were increasing age, increasing body mass index, low estimated glomerular filtration rate, Caucasian ethnicity, alcohol intake, and low left ventricular ejection fraction, while high triglycerides were associated with lower incidence. NOAF was associated with a substantial increase in the risk of adverse outcomes, with adjusted hazard ratios (HRs) of 2.01 for the composite of CV death, non-fatal myocardial infarction, or non-fatal stroke, 2.61 for CV death, 1.64 for non-fatal myocardial infarction, 2.27 for all-cause death, 8.44 for hospitalization for heart failure, and 4.46 for major bleeding (Figure 1).
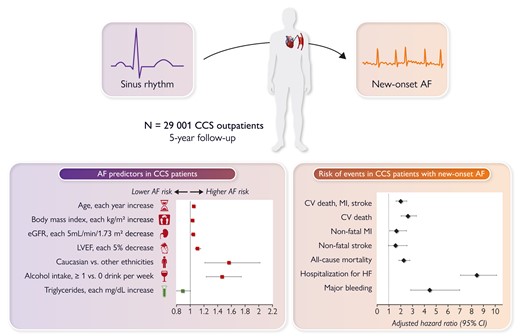
Predictors and outcomes associated with new-onset atrial fibrillation in patients with chronic coronary syndrome. AF, atrial fibrillation; CCS, chronic coronary syndrome; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction.18
The authors conclude that among CCS patients, NOAF is common and is strongly associated with worse outcomes. Whether more intensive preventive measures and more systematic screening for AF would improve prognosis in this population deserves further investigation. The contribution is accompanied by an Editorial by Robert Hatala and Peter Hlivák from the Slovak Medical University School of Medicine in Bratislava, Slovakia.19 Hatala and Hlivák commend the authors of the long-term outcome study of patients from the CLARIFY registry for their systematic efforts to better understand the prognosis of the most prevalent CAD presentation—CCS. Their findings should spark further clinical trials to expand our knowledge of the intriguing association between AF and CAD and their optimal concomitant management.
Amiodarone-related interstitial lung disease (ILD) is the most severe adverse effect of amiodarone treatment. In a Clinical Research article entitled ‘Amiodarone and pulmonary toxicity in atrial fibrillation: a nationwide Israeli study’, Gal Tsaban from the Soroka University Medical Center in Beersheva, Israel, and colleagues indicate that most data on amiodarone-related ILD are derived from periods when amiodarone was given at higher doses than currently used.20 A nationwide population-based study was conducted among patients with incident atrial fibrillation (AF) between 1999 and 2021. Amiodarone-exposed patients were matched 1:1 with controls unexposed to amiodarone based on age, sex, ethnicity, and AF diagnosis duration. The final patient cohort included only matched pairs where amiodarone therapy was consistent throughout follow-up. Directed acyclic graphs and inverse probability treatment weighting (IPTW) modelling were used. Patients with either prior ILD or primary lung cancer (PLC) were excluded. The primary outcome was the incidence of any ILD. Secondary endpoints were death and PLC. The final cohort included 6039 amiodarone-exposed patients who were matched with unexposed controls. The median age was 73 years, and 52% were women. After a mean follow-up of 4.2 years, ILD occurred in 2.0% of patients. After IPTW, amiodarone exposure was not significantly associated with ILD (HR 1.45, P = .09). There was a trivial higher relative risk of ILD among amiodarone-exposed patients between years 2 and 8 of follow-up. Primary lung cancer occurred in 0.8% of patients. After IPTW, amiodarone was not associated with primary lung cancer (HR 1.18, P = .53). All-cause death occurred in 18% of patients. After IPTW, amiodarone was associated with reduced mortality risk (HR 0.65, P < 0.001). The results were consistent across a variety of sensitivity analyses (Figure 2).
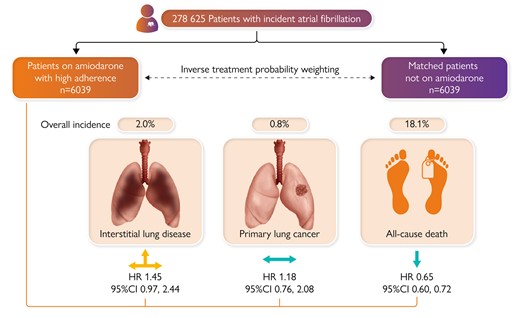
In a historical database from a large health maintenance organization, atrial fibrillation (AF) patients who were exposed to continuous amiodarone treatment had no to marginally higher risk for interstitial lung disease (ILD), similar risk for primary lung cancer (PLC), and lower risk of all-cause death compared with patients not exposed to amiodarone. The results were replicated in several sensitivity analyses including intention-to-treat and as-treated target emulation trials. CI, confidence interval; HR, hazard ratio.20
Tsaban et al. conclude that in a historical database from a large health maintenance organization, AF patients who are exposed to continuous amiodarone treatment have a marginally higher risk of ILD, similar risk of PLC, and lower risk of all-cause death compared with patients not exposed to amiodarone. This manuscript is accompanied by an Editorial by Rui Providencia and Kishore Kukendra-Rajah from the University College London in the UK, and Sergio Barra from the Hospital da Luz Arrábida in Portugal.21 The authors note that the findings of Tsaban and colleagues should be taken with a degree of caution. As the authors rightfully say, their findings will need validation by other studies. At this moment, it is premature to recommend amiodarone ≤200 mg daily in AF patients even when catheter ablation is not being contemplated. When rhythm control using a pharmacological approach is being attempted, the guidelines still recommend that owing to amiodarone’s extra-cardiac toxicity, ‘other antiarrhythmic drugs should be considered first whenever possible’.
In a Rapid Communications article entitled ‘Ventricular arrhythmias during ST-segment elevation myocardial infarction and arrhythmic complications during recurrent ischaemic events’, Marina Demidova from Lund University in Sweden, and colleagues indicate that early malignant ventricular arrhythmias (VAs) during the course of ST-elevation myocardial infarction (STEMI) markedly contribute to in-hospital mortality, yet have no influence on long-term prognosis, as the cause of arrhythmia is believed to be reversed after revascularization.22 In this study, the authors aimed to test the hypothesis that VAs during STEMI indicates an increased susceptibility to ischaemia-induced VA and is therefore associated with a higher risk of VAs during recurrent ischaemic episodes. The study cohort comprised 2148 patients discharged after primary percutaneous coronary intervention for STEMI. In total, 167 patients had re-acute coronary syndrome (re-ACS), of whom 98% were fully revascularized at index STEMI. VAs at baseline was independently associated with VAs during re-ACS [adjusted odds ratio (ORadj) 8.22; P = .007]. Ventricular arrhythmia during re-ACS was not associated with either a history of myocardial infarction prior to the index STEMI, infarct localization, the completeness of revascularization at index STEMI, or therapy at discharge.
The authors conclude that the small group of patients who are defibrillated during the early hours of STEMI and who are at a high risk of recurrent ischaemia may have a higher risk of recurrent VAs, and may therefore benefit from implantable cardioverter defibrillator therapy. More research is needed to test this hypothesis; however, these data indicate that post-STEMI sudden cardiac death risk stratification should include the presence, type, and timing of VAs, as well as the likelihood of recurrent ischaemia.
In a Viewpoint article entitled ‘Atrial fibrillation first detected after stroke: is timing and detection intensity relevant for stroke risk?’, Luciano Sposato from the Western University in London, ON (Canada), and colleagues point out that the proliferation of novel devices and their technical improvements have resulted in a steady increase in the use of prolonged cardiac monitoring (PCM) for detecting AF post-stroke in those with no history of AF.23 The rationale behind PCM post-stroke is that finding AF would warrant oral anticoagulation with the aim of preventing recurrent strokes. However, randomized controlled trials have not demonstrated a benefit of PCM for secondary stroke prevention, either by using implantable loop recording (ILR) or by repetitive Holter electrocardiograms. A randomized clinical trial comparing direct oral anticoagulants vs. antiplatelet agents in patients with a total duration of ILR-detected episodes <24 h with normal atrial size may be justified. However, some patients and physicians may be reluctant to participate, even though there is no evidence from a randomized controlled trial regarding recurrent stroke risk reduction by oral anticoagulation. Another knowledge gap is whether rhythm control therapy in AF first detected after stroke may lead to a risk reduction of recurrent stroke. Finally, in this patient population, attention should be paid to cognitive dysfunction.
The issue is also complemented by two Discussion Forum contributions. In a commentary entitled ‘Microvascular resistance reserve, does one size fit all?’, Tijn Jansen, Peter Damman, and Caïa Crooijmans from the Radboud University Medical Center in Nijmegen, the Netherlands comment on the recent publication ‘Microvascular resistance reserve: diagnostic and prognostic performance in the ILIAS registry’ by Coen K.M. Boerhout from Amsterdam UMC in the Netherlands, and colleagues.24,25 Boerhout et al. respond in a separate comment.26
The editors hope that this issue of the European Heart Journal will be of interest to its readers.
Dr. Crea reports speaker fees from Abbott, Amgen, Astra Zeneca, BMS, Chiesi, Daiichi Sankyo, Menarini outside the submitted work.
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.




