-
Views
-
Cite
Cite
Yuming Huang, Chunli Wang, Tingwen Zhou, Fei Xie, Zongtao Liu, Haiying Xu, Ming Liu, Shunshun Wang, Lanqing Li, Qingjia Chi, Jiawei Shi, Nianguo Dong, Kang Xu, Lumican promotes calcific aortic valve disease through H3 histone lactylation, European Heart Journal, Volume 45, Issue 37, 1 October 2024, Pages 3871–3885, https://doi.org/10.1093/eurheartj/ehae407
Close - Share Icon Share
Abstract
Valve interstitial cells (VICs) undergo a transition to intermediate state cells before ultimately transforming into the osteogenic cell population, which is a pivotal cellular process in calcific aortic valve disease (CAVD). Herein, this study successfully delineated the stages of VIC osteogenic transformation and elucidated a novel key regulatory role of lumican (LUM) in this process.
Single-cell RNA-sequencing (scRNA-seq) from nine human aortic valves was used to characterize the pathological switch process and identify key regulatory factors. The in vitro, ex vivo, in vivo, and double knockout mice were constructed to further unravel the calcification-promoting effect of LUM. Moreover, the multi-omic approaches were employed to analyse the molecular mechanism of LUM in CAVD.
ScRNA-seq successfully delineated the process of VIC pathological transformation and highlighted the significance of LUM as a novel molecule in this process. The pro-calcification role of LUM is confirmed on the in vitro, ex vivo, in vivo level, and ApoE−/−//LUM−/− double knockout mice. The LUM induces osteogenesis in VICs via activation of inflammatory pathways and augmentation of cellular glycolysis, resulting in the accumulation of lactate. Subsequent investigation has unveiled a novel LUM driving histone modification, lactylation, which plays a role in facilitating valve calcification. More importantly, this study has identified two specific sites of histone lactylation, namely, H3K14la and H3K9la, which have been found to facilitate the process of calcification. The confirmation of these modification sites’ association with the expression of calcific genes Runx2 and BMP2 has been achieved through ChIP-PCR analysis.
The study presents novel findings, being the first to establish the involvement of lumican in mediating H3 histone lactylation, thus facilitating the development of aortic valve calcification. Consequently, lumican would be a promising therapeutic target for intervention in the treatment of CAVD.
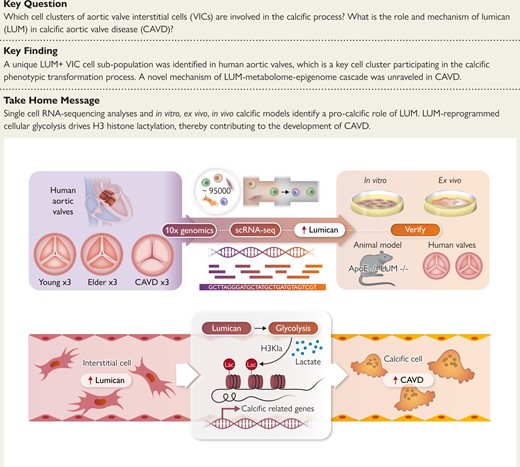
Single-cell RNA-sequencing (scRNA-seq) delineated the process of valve interstitial cell (VIC) pathological transformation and highlighted the significance of lumican (LUM) as a novel molecule in this process. The pro-calcification role of LUM was confirmed in the in vitro, ex vivo, and ApoE−/−//LUM−/− double knockout mice. The LUM induced osteogenesis in VICs via augmentation of cellular glycolysis, resulting in the accumulation of lactate. Subsequent multi-omic approaches and molecular biological verification unveiled a novel LUM driving H3 histone modification, lactylation, which plays a role in facilitating valve calcification





