-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, New light shed on the treatment of heart failure and on novel therapeutic targets, European Heart Journal, Volume 45, Issue 37, 1 October 2024, Pages 3775–3779, https://doi.org/10.1093/eurheartj/ehae643
Close - Share Icon Share
 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
This Focus Issue on heart failure and valvular heart disease contains the Fast Track Clinical Research article ‘Ferric carboxymaltose and exercise capacity in heart failure with preserved ejection fraction and iron deficiency: the FAIR-HFpEF trial’ by Stephan von Haehling from the Georg- August University in Goettingen, Germany, and colleagues.1 The authors note that iron deficiency plays an important role in several cardiovascular diseases.2–5 Yet the evidence is lacking that correcting iron deficiency (ID) has clinically important benefits for patients with heart failure with preserved ejection fraction (HFpEF). FAIR-HFpEF was a multicentre, randomized, double-blind trial designed to compare intravenous ferric carboxymaltose (FCM) with placebo (saline) in 200 patients with symptomatic HFpEF and ID (serum ferritin <100 ng/mL or ferritin 100–299 ng/mL with transferrin saturation <20%). The primary endpoint was change in 6-min walking test distance (6MWTD) from baseline to week 24. Secondary endpoints included changes in New York Heart Association class, patient global assessment, and health-related quality of life (QoL). The trial was stopped because of slow recruitment after 39 patients had been included (median age 80 years, 62% women). The change in 6MWTD from baseline to week 24 was greater for those assigned to FCM compared with placebo (least square mean difference 49 m; P = .029). Changes in secondary endpoints were not significantly different between groups. The total number of adverse events (76 vs. 114) and serious adverse events (5 vs. 19; rate ratio 0.27; P = .043) was lower with FCM than placebo.
The authors conclude that in patients with HFpEF and markers of ID, intravenous FCM improves 6MWTD and is associated with fewer serious adverse events. However, the trial lacks sufficient power to identify or refute effects on symptoms or QoL. The contribution is accompanied by an Editorial by Veraprapas Kittipibul and Robert Mentz from the Duke Clinical Research Institute, Durham, NC (USA).6 The authors note that the present modest-sized study does not allow for definitive conclusions and, while the results are promising, they should be interpreted with caution. Future randomized controlled trials should not only confirm this positive signal of i.v. iron therapy on exercise capacity but also reassess its benefits on health-related QoL and clinical endpoints, including mortality and hospitalization. A randomized controlled trial evaluating the impact of ferric derisomaltose on cardiopulmonary exercise performance (i.e. peak VO2), rather than 6MWTD, and the KCCQ score in HFpEF is underway (IRONMET-HFpEF). This is an exciting time for the field of HFpEF, with a series of recent discoveries of effective treatments (e.g. SGLT2 inhibitors and potentially GLP1-RAs) following decades of neutral or borderline results. We are hopeful that this favourable journey will continue and that i.v. iron therapy will eventually be added to the list of successes in HFpEF therapy.
Transcatheter aortic valve implantation (TAVI) has become the first choice to treat older patients with severe symptomatic aortic stenosis (AS).7–14 In a Fast Track Clinical Research article entitled ‘Transcatheter aortic valve implantation in low-risk tricuspid or bicuspid aortic stenosis: the NOTION-2 trial’, Troels Højsgaard Jørgensen from the Rigshospitalet in Denmark, and colleagues aimed to compare TAVI with surgery in low-risk patients ≤75 years of age, including both tricuspid and bicuspid AS.15 The Nordic Aortic Valve Intervention (NOTION)-2 trial enrolled and 1:1 randomized low-risk patients aged ≤75 years with severe symptomatic AS to TAVI or surgery. The primary endpoint was a composite of all-cause mortality, stroke, or rehospitalization (related to the procedure, valve, or HF) at 12 months. A total of 370 patients were enrolled with a mean age of 71 years and a median Society of Thoracic Surgeons risk score of 1.1%. A total of 100 patients had bicuspid AS. The 1-year incidence of the primary endpoint was 10.2% in the TAVI group and 7.1% in the surgery group (absolute risk difference 3.1%; hazard ratio [HR] 1.4; 95% CI, 0.7–2.9; P = .3). Patients with TAVI, when compared to surgery, had lower risk of major bleeding and new-onset atrial fibrillation and higher risk of non-disabling stroke, permanent pacemaker implantation, and moderate or greater paravalvular regurgitation. The risk of the primary composite endpoint was 8.7% and 8.3% in patients with tricuspid AS (HR 1.0) and 14.3% and 3.9% in patients with bicuspid AS (HR 3.8) treated with TAVI or surgery, respectively (P for interaction = .1).
The authors conclude that among low-risk patients aged ≤75 years with severe symptomatic AS, the rate of the composite of death, stroke, or rehospitalization at 1 year is similar between TAVI and surgery. Transcatheter aortic valve implantation outcomes in young bicuspid AS patients warrant caution and should be further investigated. The contribution is accompanied by an Editorial by Niklas Schofer and Stefan Blankenberg from the University Medical Center Hamburg-Eppendorf in Hamburg, Germany.16 The authors highlight that given the current evidence the choice of treatment modality for younger patients with AS should be based on individual risk–benefit assessment taking into consideration patients’ life expectancy, anatomy, and medical treatment. The present trial cautiously looks to the future to pave the way towards TAVI as the first treatment choice even in younger patients below 75 years of age. Without any doubt, persuasive 10-year results are needed to establish the foundation for the ultimate step in this direction. The trial further raises concerns for TAVI treatment in bicuspid AS patients and informs the community about potential pitfalls in future trial designs targeting the treatment of bicuspid AS patients. Finally, an ageing society, which will increasingly face non-communicable diseases such as AS, needs to invest in independent medical strategy trials to determine the best medical approach.
In a Fast Track Clinical Research article entitled ‘Sex-related differences in severe native valvular heart disease: the ESC-EORP Valvular Heart Disease II survey’, Julia Mascherbauer from the University Hospital St Pölten in Austria, and colleagues assess sex differences in disease characteristics and treatment of patients with severe native valvular heart disease (VHD) included in the VHD II EURObservational Research Programme.17 A total of 5219 patients were enrolled in 208 European and North African centres and followed for 6 months (41% AS, 5.3% aortic regurgitation [AR], 4.5% mitral stenosis [MS], 21% mitral regurgitation [MR], 2.7% isolated right-sided VHD, 25% multiple left-sided VHD). Indications for intervention were considered concordant if corresponding to class I recommendations specified in the 2012 ESC or 2014 AHA/ACC VHD guidelines. Overall, women were older, more symptomatic, and presented with a higher EuroSCORE II. Bicuspid aortic valve and AR were more prevalent among men while mitral disease, concomitant tricuspid regurgitation (TR), and AS above age 65 were more prevalent among women. On multivariable regression analysis, concordance with recommended treatment was significantly poorer in women with MS and primary MR (both P < .001). Age, patient refusal, and decline of symptoms after conservative treatment were reported significantly more often as reasons to withhold the intervention in females. Concomitant tricuspid intervention was performed at a similar rate in both sexes although prevalence of significant TR was considerably higher in women. In-hospital and 6-month survival did not differ between sexes (Figure 1).
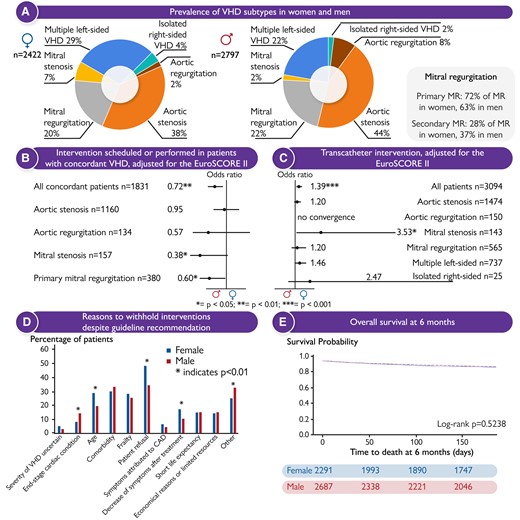
(A) Valvular heart disease (VHD) subtypes stratified by sex; (B) forest plots displaying women’s odds ratios for ‘Intervention scheduled or performed’ and (C) ‘Transcatheter intervention’ in patients with concordant VHD, adjusted for the EuroSCORE II; (D) reasons to withhold interventions despite guideline recommendation; (E) Kaplan–Meier curves for overall survival at 6 months, stratified by sex. CAD, coronary artery disease; MR, mitral regurgitation.17
Mascherbauer et al. conclude that VHD subtype varies between sexes. They also note that concordance with recommended intervention for MS and primary MR is significantly lower for women, and that survival of men and women is similar at 6 months. The contribution is accompanied by an Editorial by Patrizio Lancellotti from the University of Liège Hospital in Belgium, Yun Yun Go from the National Heart Centre Singapore, and Mani A Vannan from the Piedmont Heart Institute in Atlanta, GA (USA).18 The authors note that three decades after Dr Bernadine Healy first described the Yentl syndrome the cardiology community has made progress in diagnosing and managing ischaemic heart disease in women. However, gender data gaps still exist in many areas of cardiology, including VHD, HF, arrhythmias, and cardiometabolic disease; thus, closing the gender gaps in cardiovascular research remains work in progress. Ultimately, we strive for equitable healthcare, where sex or gender equality is an important but not the sole determinant. The holy grail should be the representation of women (and men) of different races, ethnicities, and socioeconomic backgrounds in cardiovascular research to help us better understand health behaviours and needs at the intersections of these identity dimensions.
Many patients are prescribed loop diuretics without a diagnostic record of HF. In a Clinical Research article entitled ‘Loop diuretic therapy with or without heart failure: impact on prognosis’, Jocelyn Friday from the University of Glasgow in the United Kingdom, and colleagues point out that little is known about their characteristics and prognosis.19 Glasgow regional health records (2009–16) were obtained for adults with cardiovascular disease or taking loop diuretics. Outcomes were investigated using Cox models with HRs adjusted for age, sex, socioeconomic deprivation, and comorbid disease (adjHR). Of about 200 000 patients (median age 65 years; 55% women), 81% neither took loop diuretics nor had a diagnostic record of HF (reference group), 12% were taking loop diuretics but had no HF recorded, 4% had HF recorded and took loop diuretics, and 3% had HF recorded but were not receiving loop diuretics. Compared with the reference group, 5-year mortality was only slightly higher for HF in the absence of loop diuretics (22%; adjHR 1.2), substantially higher for those taking loop diuretics with no record of HF (40%; adjHR 1.8), and highest for HF treated with loop diuretics (52%; adjHR 2.2) (Figure 2).
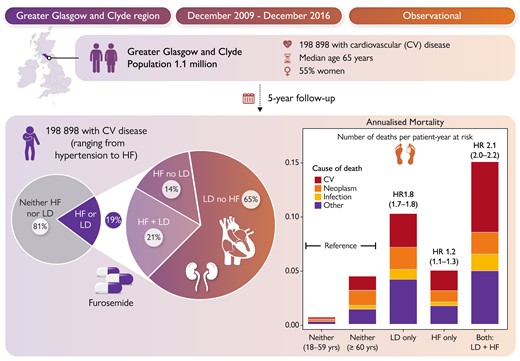
The authors conclude that for patients with cardiovascular disease, many are prescribed loop diuretics without a recorded diagnosis of HF. Mortality is more strongly associated with loop diuretic use than with a record of HF. The diagnosis of HF may often be missed, or loop diuretic use is associated with other conditions with a prognosis similar to HF, or inappropriate loop diuretic use increases mortality; all might be true. This manuscript is accompanied by an Editorial by Annika Rosengren from Sahlgrenska University Hospital in Gothenburg, Sweden.20 Rosengren concludes that with higher awareness of HF among clinicians, wider use of natriuretic peptides for diagnostic purposes, and with modern treatment, patients who do not need diuretics are likely to show a slightly different phenotype than in classical HF where congestion is a presenting feature. Importantly, their relatively favourable prognosis should lead to an increased awareness among clinicians about HF as a potential cause of vague and non-specific symptoms of fatigue, deconditioning, and palpitations, prompting a careful evaluation and laboratory tests, in particular in the presence of risk factors for HF such as coronary heart disease, hypertension, diabetes, or obesity. Among patients on loop diuretic treatment lacking a clear indication, their continuing use should be carefully evaluated, which in many cases could mean added diagnostic work-up. Also, because symptoms and signs may be both non-specific and at the same time not sensitive enough, it should be possible to identify as well as define HF at an earlier stage, through a more liberal use of measurements of natriuretic peptides, interpreted in the context of the clinical setting, and followed by cardiac imaging, if needed
HF is a leading cause of mortality worldwide and is characterized by significant co-morbidities and dismal prognosis.21–24 Neutrophil extracellular traps (NETs) aggravate inflammation in various cardiovascular diseases. Their function and mechanism of action in HF pathogenesis remain underexplored. In a Translational Research article entitled ‘Von Willebrand factor exacerbates heart failure through formation of neutrophil extracellular traps’, Ge Mang from The Second Affiliated Hospital of Harbin Medical University in China, and colleagues investigated the involvement of a novel VWF-SLC44A2-NET axis in HF progression.25 NET levels were examined in patients with HF and mouse models of transverse aortic constriction (TAC) HF. PAD4 knockout mice and NET inhibitors (GSK-484, DNase I, NEi) were used to evaluate the role of NETs in HF. RNA sequencing was used to investigate the downstream mechanisms. Recombinant human ADAMTS13 (rhADAMTS13), ADAMTS13, and SLC44A2 knockouts were used to identify novel upstream factors of NETs. Elevated NET levels were observed in patients with HF and TAC mouse models of HF. PAD4 knockout and NET inhibitors improved the cardiac function. Mechanistically, NETs induced mitochondrial dysfunction in cardiomyocytes, inhibiting mitochondrial biogenesis via the NE-TLR4-mediated suppression of PGC-1α. Furthermore, VWF/ADAMTS13 regulated NET formation via SLC44A2. Additionally, sacubitril/valsartan amplified the cardioprotective effects of the VWF-SLC44A2-NET axis blockade.
The authors conclude that this study establishes the role of a novel VWF-SLC44A2-NET axis in regulating mitochondrial homeostasis and function, leading to cardiac apoptosis and contributing to HF pathogenesis. Targeting this axis may offer a potential therapeutic approach for HF treatment. The contribution is accompanied by an Editorial by Stefano Ministrini and Amedeo Tirandi from the University of Zurich in Schlieren, Switzerland.26 The authors note that additional research is warranted to confirm these experimental observations, especially regarding the role of Slc44A2 as neutrophils receptor of vWF, the possible pleiotropic effects of sacubitril/valsartan, and the pharmacological relevance of the proposed interaction between sacubitril/valsartan and Slc44A2. Indeed, in these aspects, the authors provided only associative evidence, mainly derived by computational studies. Should these observations be confirmed, they would pave the way to novel treatment for HF based on the modulation of the vWF/ADAMTS13 axis, for instance by administration of recombinant ADAMTS13, as already proposed for myocardial infarction, acute ischaemic stroke, and chronic kidney disease.
Valve interstitial cells (VICs) undergo a transition to intermediate state cells before ultimately transforming into the osteogenic cell population, which is a pivotal cellular process in calcific aortic valve disease (CAVD). In a Translational Research article entitled ‘Lumican promotes calcific aortic valve disease through H3 histone lactylation’, Yuming Huang from The First Affiliated Hospital of Nanjing Medical University in China, and colleagues successfully delineated the stages of VIC osteogenic transformation and elucidated a novel key regulatory role of lumican (LUM) in this process.27 Single-cell RNA-sequencing (scRNA-seq) from nine human aortic valves was used to characterize the pathological switch process and identify key regulatory factors. The in vitro, ex vivo, in vivo, and double knockout mice were constructed to further unravel the calcification-promoting effect of LUM. Moreover, the multi-omic approaches were employed to analyse the molecular mechanism of LUM in CAVD. ScRNA-seq successfully delineated the process of VIC pathological transformation and highlighted the significance of LUM as a novel molecule in this process. The pro-calcification role of LUM was confirmed on the in vitro, ex vivo, in vivo level, and ApoE−/−//LUM−/− double knockout mice. The LUM induced osteogenesis in VICs via activation of inflammatory pathways and augmentation of cellular glycolysis, resulting in the accumulation of lactate. Subsequent investigation unveiled a novel LUM driving histone modification, lactylation, which plays a role in facilitating valve calcification. More importantly, this study identified two specific sites of histone lactylation, namely, H3K14la and H3K9la, which were found to facilitate the process of calcification. The confirmation of these modification sites’ association with the expression of calcific genes Runx2 and BMP2 was achieved through ChIP-PCR analysis.
The authors conclude that the study presents novel findings, being the first to establish the involvement of LUM in mediating H3 histone lactylation, thus facilitating the development of aortic valve calcification. Consequently, LUM would be a promising therapeutic target for intervention in the treatment of CAVD. The manuscript is accompanied by an Editorial by François Mach and Kapka Miteva from the Geneva University Hospital and Faculty of Medicine, and colleagues.28 Mach and Miteva conclude by noting that the study by Huang et al. presents a novel insight into the initial stage of the pathological alterations in CAVD and opens up an opportunity for the development of an effective early medical intervention for CAVD targeting LUM and lactylation shown to potentially regulate calcification-related genes.
In a Rapid Communications contribution entitled ‘Dapagliflozin, peptide YY, and weight loss in heart failure with preserved ejection fraction’, Yogesh NV Reddy from the Mayo Clinic in Rochester, MN (USA), and colleagues note that although sodium–glucose cotransporter 2 inhibitors (SGLT2i) cause glycosuria and natriuresis, the diuretic effect upon initiation is mild, transient, and cannot explain the magnitude of observed long-term weight loss.29 Among patients with HFpEF, there is greater weight loss with dapagliflozin in obese compared witho lean individuals, and most of the weight reduced is fat, rather than water or lean tissue. These observations remain poorly understood. A total of 34 participants with HFpEF had paired arterial blood samples available for proteomic analysis (14 placebo, 20 dapagliflozin). One hundred and ninety proteins demonstrated nominally significant changes at an uncorrected P < .05, but after correction for false discovery, there was a significant change in only one protein: peptide YY (PYY), a hormone released by cells in the distal intestine in response to feeding that promotes satiety. As compared with placebo, dapagliflozin increased PYY at 24 weeks (corrected P = .034). To confirm the proteomic findings, the authors then measured PYY by ELISA in independent paired arterial samples in a blinded fashion. The median PYY level at baseline was 102.4 pg/mL. After 24 weeks, dapagliflozin increased PYY by +31.9 pg/mL compared with a change of −6.2 pg/mL with placebo (P = .02). Dapagliflozin resulted in a placebo-corrected weight loss of 3.4 kg (P = .01) in the 34 patients with paired proteomic analyses. Increases in PYY on treatment were correlated with greater weight loss (P = .003), including greater reduction in trunk fat (P < .0001) and android fat (P = .005). In addition, dapagliflozin enhanced pulmonary vascular reserve with exercise and, notably, increases in PYY were correlated with greater improvements in pulmonary artery compliance (P = .0009) and decreases in elastance (P = .007) during 20 W exercise.
The authors conclude that treatment with dapagliflozin for 24 weeks increases plasma PYY levels, and the extent of this increase is correlated with weight loss, particularly adipose tissue loss, and favourable haemodynamic effects during exercise. These data raise the possibility that SGLT2i-mediated increases in PYY may contribute to weight loss in obese HFpEF through increased satiety, with secondary haemodynamic benefits.
The issue is also complemented by two Discussion Forum contributions. In a commentary entitled ‘More questions than answers after NOTION-2’, Victor Dayan from the Universidad de la Republica in Montevideo, Uruguay, and colleagues comment on the recent publication ‘Transcatheter aortic valve implantation in low-risk tricuspid or bicuspid aortic stenosis: the NOTION-2 trial’ by Troels Højsgaard Jørgensen from the University Hospital in Copenhagen, Denmark, and colleagues.30,15 Jørgensen et al. respond in a separate comment.31
The editors hope that this issue of the European Heart Journal will be of interest to its readers.
Dr. Crea reports speaker fees from Abbott, Amgen, Astra Zeneca, BMS, Chiesi, Daiichi Sankyo, Menarini outside the submitted work.
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.




