-
PDF
- Split View
-
Views
-
Cite
Cite
Florian Schlotter, Kurt Huber, Christian Hassager, Sigrun Halvorsen, Pascal Vranckx, Janine Pöss, Konstantin Krychtiuk, Roberto Lorusso, Nikolaos Bonaros, Patrick A Calvert, Matteo Montorfano, Holger Thiele, Ventricular septal defect complicating acute myocardial infarction: diagnosis and management. A Clinical Consensus Statement of the Association for Acute CardioVascular Care (ACVC) of the ESC, the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC and the ESC Working Group on Cardiovascular Surgery, European Heart Journal, Volume 45, Issue 28, 21 July 2024, Pages 2478–2492, https://doi.org/10.1093/eurheartj/ehae363
Close - Share Icon Share
Abstract
Ventricular septal defects are a rare complication after acute myocardial infarction with a mortality close to 100% if left untreated. However, even surgical or interventional closure is associated with a very high mortality and currently no randomized controlled trials are available addressing the optimal treatment strategy of this disease. This state-of-the-art review and clinical consensus statement will outline the diagnosis, hemodynamic consequences and treatment strategies of ventricular septal defects complicating acute myocardial infarction with a focus on current available evidence and a focus on major research questions to fill the gap in evidence.
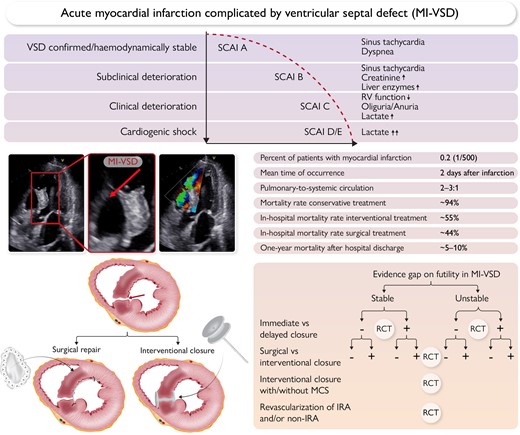
Top panel: Clinical course of ventricular septal defect complicating acute myocardial infarction.
Middle panel: left: Echocardiographic visualization of a myocardial infarction ventricular septal defect (MI-VSD); right: major clinical characteristics of MI-VSD.
Lower panel: left: MI-VSD treatment by surgery or percutaneous device closure; right: gaps in evidence and potential clinical trial design in MI-VSD.
IRA, infarct-related artery; MCS, mechanical circulatory support; RCT, randomized controlled trial; SCAI, Society for Cardiovascular Angiography and Interventions shock stage classification.
Introduction
Short- and long-term outcomes after acute myocardial infarction (AMI) have improved due to advanced treatment options, reducing heart failure syndromes, cardiogenic shock (CS), and cardiovascular events.1–3 However, mechanical complications remain a major concern, increasing morbidity and mortality.4–7 These include acute mitral regurgitation, free wall rupture, and ventricular septal defect (VSD).8 VSD after myocardial infarction (MI-VSD) has a dismal prognosis, approaching 100% fatality if treated conservatively.9 It results from interventricular septal rupture, leading to left and right ventricular (LV and RV) communication. MI-VSD has profound hemodynamic effects, causing heart failure, pulmonary edema, and CS. Diagnosis is challenging, necessitating clinical, electrocardiographic, echocardiographic, and hemodynamic assessments.
Managing MI-VSD demands a multidisciplinary approach. Early recognition and intervention, ideally before CS develops, are essential. Medical management aims to stabilize the patient, optimize hemodynamics, and reduce myocardial workload. Surgical repair or interventional closure remain the definitive treatment for MI-VSD, but still are associated with excessively high mortality.9–12 Despite the availability of these technically challenging interventions, MI-VSD continues to pose a significant clinical dilemma. Available observational data to guide clinical decision-making are limited by the small size of the studies as well as the selection and survival bias, while randomized data are lacking,13,14 and limitations in the treatment options due to the high level of invasiveness and frequent residual shunt, irrespective of the applied closure technique.13 In addition, most available observational studies do not consider mechanical circulatory support (MCS) in this special patient population.
The complex hemodynamic derangements, ongoing ischemia, and underlying ventricular dysfunction contribute to the high mortality rates associated with this condition. Understanding the challenges and exploring potential therapeutic interventions is essential for optimizing outcomes in patients with MI-VSD. Identifying optimal strategies, refining patient selection, and intervention timing are ongoing research priorities. This consensus document synthesizes existing literature based on a structured selective search to offer clinicians insights, evidence-based and expert advice, and urgent future perspectives for managing MI-VSD.
Epidemiology and clinical burden of MI-VSD
The incidence of MI-VSD was approximately 1–3% in the pre-reperfusion era, but has declined significantly after the introduction of modern reperfusion therapy.15 Recent reports have found an incidence of MI-VSD between 0.17% and 0.44%.7,15,16 A recent US study, assessing more than 9 million hospitalizations for AMI, reported an incidence of VSD of 0.21% of ST-elevation myocardial infarction (STEMI) and 0.04% of non-ST-elevation myocardial infarction (NSTEMI) patients.5 Of note, the incidence has not changed during the past 20 years.5
The time from infarction to ventricular rupture is typically 48 h,15 but may be up to 2 weeks. The perforation ranges from one to several centimeters in length, and may be anterior or posterior. Anterior MI-VSDs are usually caused by infarcts in the left anterior descending artery territory, whereas posterior MI-VSDs are caused by inferior infarcts. The size of the defect determines the magnitude of left-to-right shunting, which in turn affects prognosis. Morphologically, MI-VSDs may be direct or serpiginous, or in rare cases multifenestrated.17,18
Risk factors for MI-VSD include older age, female sex, chronic kidney disease, anterior infarction, and delayed reperfusion.6
In the Mechanical Complications of Acute Myocardial Infarction: an International Multicenter Cohort (CAUTION) study of patients who were treated surgically for mechanical complications of AMI, 90% of the MI-VSDs occurred in the setting of STEMI, while only 14% of MI-VSD patients had undergone previous revascularization procedures.19 If treated conservatively, unpredictable hemodynamic deterioration occurs in most patients in the days and weeks following MI-VSD. In these conservatively treated cases, the fatality rate approaches 100% (Graphical Abstract).7,9
Diagnostic approaches and challenges
The typical clinical presentation of patients with MI-VSD is acute-onset heart failure or CS with the clinical signs of hypoperfusion, such as cold-sweated and mottled skin, prolonged capillary refill time, mental confusion, and reduced urine output. Initial symptoms typically include dyspnoea and orthopnoea. Recurrent angina or new ST-segment changes may occur. The clinical presentations range from an incidental murmur to CS with circulatory collapse. The left-to-right shunt usually produces a holosystolic murmur along the left parasternal border. Therefore, patients with AMI should undergo clinical examination, including cardiac auscultation, regularly. However, the murmur may be very difficult to detect in the setting of extensive shunting or severe CS with pulmonary oedema. Signs of right heart failure, like jugular venous distension, frequently develop. Laboratory analyses might show signs of end-organ (renal/liver) failure and might reveal increased arterial lactate levels and low arterial oxygen levels in more severe cases.15,18 Suspicion of a mechanical complication of AMI should prompt immediate clinical evaluation. Subacute infarct presentation should raise the level of suspicion further. MI-VSD, like any other mechanical complication post AMI, should be suspected in the presence of hemodynamic instability that is not consistent with the extent of ventricular dysfunction, ECG changes and/or the level of cardiac enzyme elevation.
Transthoracic echocardiography (TTE) with Doppler imaging is the initial investigation of choice and it is diagnostic in the vast majority of cases. Since conventional 2D TTE alone may only show suggestive signs, such as thinned or focally absent myocardia tissue at the level of the septum, use of colour flow Doppler to document the shunt through the septum is crucial. The optimal window in the acute dyspnoeic patient is often the subcostal view but it is advised that all windows should be approached, focusing on the ventricular septum if a patient with AMI develops a systolic murmur or deteriorates hemodynamically (Figure 1A and B).8
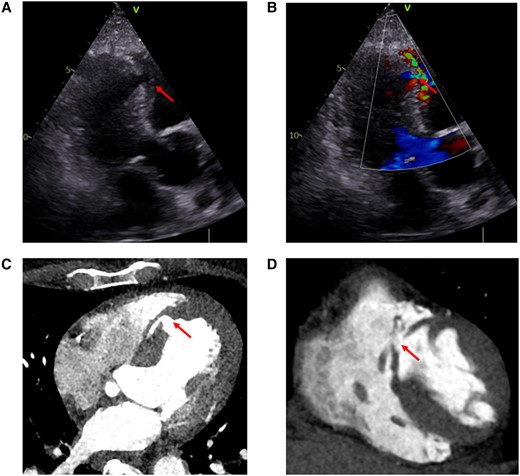
Anterior MI-VSD (arrows). (A and B) Transthoracic echocardiogram (apical long-axis view). (A) 2D echocardiography; (B) color flow Doppler. (C, D) Computed tomography scans
Echocardiographic assessment of VSD includes: location, size, and morphology of the defect(s), delineation of adjacent anatomical structures, shunt quantification/estimation and evaluation of hemodynamic significance, LV and RV function, pulmonary artery pressure, and cardiac output (CO). Anterior MI-VSDs tend to be simpler and usually involve the apical septum, while MI-VSDs associated with inferior wall AMI more often involve the basal septum or even the right ventricle and may be more complex (serpiginous or with multiple fissures). Besides diagnosing the MI-VSD, TTE is crucial to detect important differential diagnoses, which sometimes might even co-exist, such as acute mitral valve regurgitation due to papillary muscle rupture or (contained) free-wall rupture.
Transesophageal echocardiography (TEE) may be helpful in cases with a poor acoustic window and is usually used intraoperatively or during interventional treatment as guidance. Notably, the echocardiographic examination must be performed thoroughly because it is the basis for decisions regarding the further treatment of the patient (indication, feasibility, and technique of closure). ECG-gated computed tomography (CT) or cardiac magnetic resonance (CMR) scans are helpful for confirming the diagnosis and for determining the exact anatomical characterization of the MI-VSD before planning interventional or surgical closure (Figure 1C and D). In the catheterization laboratory, MI-VSD can be diagnosed by left ventriculography in conjunction with coronary angiography. Right heart catheterization can be helpful for diagnosis in rare cases by demonstrating the increase in oxygen saturation that occurs within the right ventricle. Furthermore, it allows for shunt quantification.20 The extent of left-to-right shunting is commonly evaluated echocardiographically or by right heart catheterization and typically is in the range of Qp:Qs ∼2–3:1 (see Supplementary data online, Table S1).21,22 A general diagnostic approach is displayed in Figure 2.
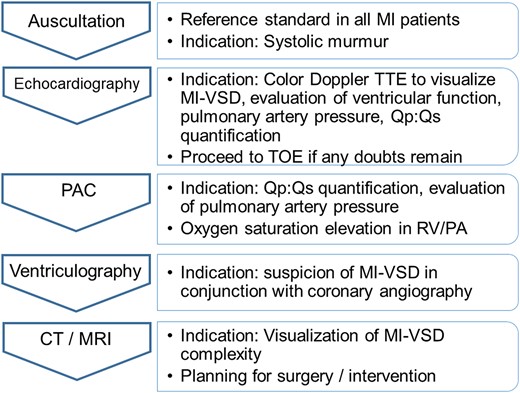
Diagnostic approach. CT, computed tomography; MI, myocardial infarction; MI-VSD, myocardial infarction ventricular septal defect; MRI, magnetic resonance imaging; PA, pulmonary artery; PAC, pulmonary artery catheterization; RV, right ventricle; Qp, pulmonary blood flow; Qs, systemic blood flow
Some limitations of the diagnostic modalities need to be mentioned: TTE may have limited sensitivity to detect MI-VSD in some cases. While TEE may provide superior imaging in case of poor acoustic windows with TTE, sedation with potential hemodynamic compromise may be necessary. MRI may be challenging as the extended procedure time may not be tolerated hemodynamically.
Hemodynamic consequences of MI-VSD
The hemodynamic effects of MI-VSD are significant and contribute to the clinical severity and poor prognosis. One of the key hemodynamic consequences of MI-VSD is the development of a ventricular left-to-right shunt. The hemodynamic significance is influenced by several factors, including defect size, RV and LV pressures and function, and pulmonary as well as systemic vascular resistance.23 The left-to-right shunting leads to significant hemodynamic disturbances characterized by increased pulmonary artery pressures and blood flow, RV failure, elevated right atrial and central venous pressures, a reduction in CO and elevated pulmonary capillary wedge pressures (PCWP) (see Supplementary data online, Table S1).9,21,22,24 This results in reduced systemic arterial pressures and inadequate tissue perfusion. The severity of hemodynamic compromise in MI-VSD is further exacerbated by the associated LV and RV dysfunction. The presence of LV dysfunction, commonly seen in the context of AMI, contributes to decreased CO and impaired forward flow. RV dysfunction, which may result from the increased RV overload due to the shunting, further compromises cardiac function.
Medical, interventional, and surgical treatment approaches
Medical treatment
When forward flow declines, compensatory vasoconstriction leads to increasing systemic vascular resistance, which may worsen left-to-right shunting. Afterload reduction in patients with preserved blood pressure may reduce left-to-right shunting and improve CO, but may also cause hypotension. Patients with hypotension are usually treated with inotropes and vasopressors to maintain tissue perfusion and arterial blood pressure even though it may increase the shunt fraction. To increase CO, short-acting inotropes, like dobutamine, or milrinone may be utilized and the hemodynamic response should be monitored closely. To address hypotension, preferably norepinephrine is used. However, prediction of the hemodynamic response to therapy with vasopressors or inodilators in MI-VSD is challenging. In patients with adequate blood pressure and high systemic vascular resistance, short-acting vasodilators, like nitroglycerine may be used to lower systemic vascular resistance and thereby influence the shunt flow ratio. All medical therapy should be titrated up from low initial levels and stopped if hemodynamics worsen. Any attempt to stabilize the patient’s condition with medical therapy is always only temporary until an intervention with VSD closure or upscale to MCS, as a bridge, can be done. Oxygenation must be maintained with the administration of oxygen by mask or intubation with mechanical ventilation in severe cases. Endotracheal intubation and mechanical ventilation may become necessary but may have deleterious hemodynamic effects in LV dysfunction and especially CS. MI-VSD complicates the situation and the hemodynamic response following mechanical ventilation is difficult to anticipate.
Surgical treatment
The selection of a surgical approach for MI-VSD repair should consider the following aspects: (1) operative risk, based on the hemodynamic and end-organ condition as well as MI-VSD dimension and localization; (2) patients’ age and comorbidities; (3) RV and LV function and prediction of the probability of further LV or RV or biventricular function deterioration after surgery; (4) type of surgical technique; (5) need for concomitant coronary revascularization; (6) need for MCS to treat perioperative low CO syndrome; (7) risk of VSD recurrence; (8) local expertise on advanced cardiac support.
The surgical techniques considered for MI-VSD are based on the localization, defect size, extent of ventricular dysfunction and local expertise. Ventricular apical amputation may be appropriate for apical defects. For mid- or basal septum MI-VSDs the ‘infarct excision’ (Daggett) and the ‘infarct exclusion’ (David) technique can be used.25–27 In both procedures, the defect is approached via an anterior incision for anterior or an inferior incision for posterior VSDs. Alternative approaches include a trans-mitral or trans-tricuspid valve access to avoid further injury of ventricular myocardium.25,27,28
The ‘excision technique’ includes resection of the residual septal necrotic tissue by single patch reconstruction of the septum sutured directly along the edge of the VSD.25 The ‘exclusion technique’ consists of the use of pericardial or prosthetic material for the creation of a new ventricular septum with the autologous or prosthetic patch sutured far from the necrotic VSD edge at the non-infarcted part of the LV-related site of the septum.27 A recent meta-analysis based on observational data suggested a trend towards lower operative mortality for the ‘exclusion technique’.28
The risk of residual VSD due to the fragile necrotic septal tissue led to the development of ‘enforced’ closure techniques.29 The ‘sandwich technique’, includes the placement of two patches sutured on both septal sides along with glue application between them.30 Another version of this approach combines the ‘excision’ and the ‘exclusion’ techniques by using a smaller patch for direct VSD closure and a second, larger patch to re-shape the left ventricle.31 A further evolution represents the ‘triple-patch technique’, in which a smaller patch is used to close the defect and two large patches are used to exclude both ventricles.32 The main surgical techniques are depicted in Figure 3.
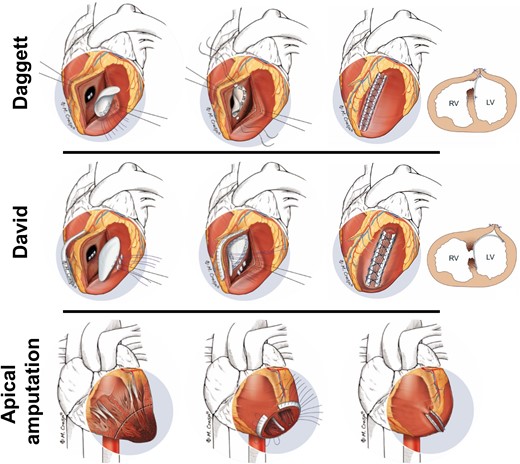
Surgical techniques of MI-VSD treatment: For mid- or basal septum MI-VSDs the ‘infarct excision’ (Daggett) and the ‘infarct exclusion’ (David) technique can be used. Ventricular apical amputation may be appropriate for apical defects
Interventional treatment
Transcatheter closure of MI-VSD was first described in 198833 and progressively became an alternative to surgical interventions in select cases. Indeed, most of the MI-VSDs present anatomical features suitable for percutaneous closure, with few theoretical exclusion criteria (VSD size of >35 mm, apical VSD without a suitable rim and basal VSD too close to the valvular apparatus, serpiginous VSD pattern).12 However, transcatheter closure can be technically demanding and requires meticulous planning. Several septal occluders have been reported in the literature, with the Amplatzer P.I. Muscular VSD being the only specifically designed for MI-VSD and the most studied.13,34
The procedure is usually performed under TEE guidance. The VSD is usually crossed from the left side, with following snaring of the wire in the pulmonary artery from a venous access (femoral in case of anterior defects and jugular in basal defects) to create an arterio-venous circuit (Figure 4A and B).

(A, B) Schematic reconstruction of the arterio-venous circuit. (C) CT scan may help in MI-VSD visualization (size, morphology) and in predicting fluoroscopic projection to easily cross the defect. Fluoroscopic projection derived from CT scan (cranial 19°, left anterior oblique 31°). CT, computed tomography; PA, pulmonary artery; RV, right ventricle; LV, left ventricle; MI-VSD, myocardial infarction ventricular septal defect
Practically, the size of the device is chosen with at least 3 mm oversizing according to echocardiographic measurements. The device is than advanced from the right side and delivered through 9 or 10 Fr long sheaths.35 It can be useful to leave a 0.018 inch wire through the defect in the left ventricle in case of mispositioning of the device during the first attempt, helping in the recrossing of the defect from the right side. A single arterial access technique with avoidance of the arterio-venous circuit has also been reported.36 Procedural planning is of paramount importance: CT or MRI helps in defining VSD morphology and dimension (which can differ significantly between systole and diastole; Figure 4C).37 Moreover, it can help in predicting the precise fluoroscopic projection to cross the VSD (Figure 4).
Nevertheless, further technical advancements and device iterations are eagerly required to address the current limitations of this technique including non-availability of a larger range of device sizes, often incomplete VSD closure, device embolization, and requirement of an arterio-venous circuit.
Outcomes of percutaneous MI-VSD closure has been recently reported in a large series of the UK national registry.34 Among more than 130 patients (half of them presenting with CS), a single device was effectively implanted in 85%, with partial shunt reduction in 70% of patients and complete reduction in 20%. No shunt reduction was achieved in the remaining 10% of patients. However, the rate of device embolization (8%) was not negligible, and 13% of patients with an initial percutaneous management required an additional procedure (surgical or percutaneous). In-hospital mortality was high (55%). Importantly, the creation of the arterio-venous circuit and the need to pass the fragile defect with stiff wires and the delivery sheath may enlarge the defect and lead to further hemodynamic deterioration.
A hybrid transcatheter/surgical repair has recently been suggested for apical MI-VSDs, omitting some of the challenges of a transcatheter procedure as well as sterno- and ventriculotomy.38
Figure 5 provides a summary of the treatment options and associated mortality rates. Of note, selection and survival bias likely play a major role. Complications, specific to either surgical or interventional repair are summarized in Supplementary data online, Table S2.
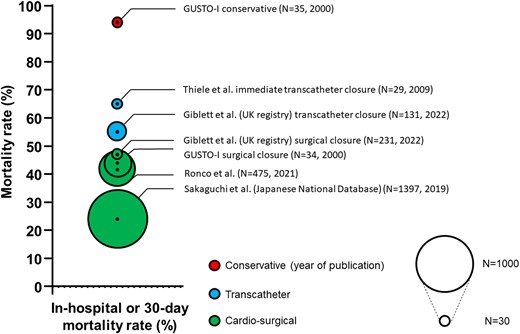
Treatment strategies and associated 30-day or in-hospital mortality of select registries and reports
Mechanical circulatory device therapy in MI-VSD
Temporary mechanical circulatory device therapy in MI-VSD
In patients with MI-VSD, a well-timed closure is recommended in the 2023 European acute coronary syndrome guidelines.8 Especially in patients without end-organ failure, a delayed treatment may allow for connective tissue or scar formation around the defect, resulting in a better anchor for devices and for suture material, resulting in a lower risk for patch or occluder dehiscence and VSD recurrence/persistence. Nonetheless, the time required for significant ‘firming’ of tissues (>2 weeks) is likely to be greater than is practical to maintain a patient on MCS. While waiting for surgery or transcatheter intervention, the cornerstone of MI-VSD management is LV afterload reduction to decrease left-to-right shunting. In patients developing CS, prompt initiation of patient-tailored temporary MCS may reverse or prevent irreversible multiorgan damage and hemodynamic collapse, and bridge patients towards definitive therapies.15,39 The utilization of MCS for addressing mechanical complications, with the aim of improving pre-operative clinical and hemodynamic conditions reflects a recent development in MI-VSD management. Nevertheless, the effectiveness of this approach necessitates further research and evidence to ascertain whether it delivers a clinical advantage.8 According to current ESC guidelines, intra-aortic balloon pumping (IABP) should be considered for patients to bridge to definitive therapy.8 However, the level of evidence remains at expert opinion.8 Recent data show no benefit of MCS by veno-arterial extracorporeal membrane oxygenation (VA-ECMO) in patients with CS secondary to AMI.40,41 However, no randomized controlled trial (RCT) is available for MCS therapy in patients with mechanical complications of AMI including MI-VSD.
Several MCS strategies are available.42 Devices distinguish themselves in terms of insertion technique, sites where blood is withdrawn and returned to the body, flow capacities, and pumping mechanisms.42 The ideal configuration of MCS in MI-VSD may be identified considering the individual patient’s characteristics including VSD specifics (i.e. size, location, pressure gradient and direction of flow across the defect), RV function, accompanying cardiovascular pathologies (e.g. peripheral artery disease, valvular heart disease or LV thrombi), and respiratory function. These characteristics provide information about the required degree of uni- or biventricular hemodynamic support, about contraindications for certain devices, and whether or not respiratory support is necessary.6,43 Each MCS device almost invariably influences VSD-related hemodynamics (i.e. pulmonary and systemic vascular resistance).39,44 The interactions between the diseased heart, the vasculature and the device are subject to multivariable assumptions and treatment responses are hard to predict. Understanding the pathophysiology of these changes is critical for proper monitoring, troubleshooting, and assessment of device performance.39,42,44
According to the 2023 European acute coronary syndrome guidelines, IABP support represents the first-line MCS in patients with MI-VSD.8 Conceptually, an IABP reduces LV afterload and LV wall stress, thereby facilitating LV contractility and increased CO, simultaneously reducing left-to-right shunting (Figure 6).44,45 However, its hemodynamic support effect can be insufficient in case of severe hemodynamic compromise. IABP use has also been suggested as LV venting during VA-ECMO support.46,47
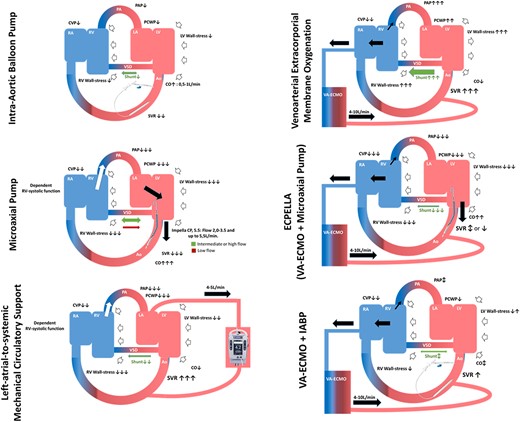
Device specific hemodynamic effects of MCS on the underlying myocardial infarction VSD pathophysiology. For flow-variable supports (i.e. VA-ECMO, microaxial pump, left-atrial-to-systemic MCS and LVAD) the effects on the shunt may be controlled and partially compensated by adjusting the relative flow intensity of each device. Ao denotes aorta; CO, cardiac output; CVD, central venous pressure, IABP, intra-aortic balloon pump; LA, left atrium; LV, left ventricle, PA, pulmonary artery; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RA, right atrium; RV, right ventricle; SVR, systemic vascular resistance; (C)VA-ECMO, (central) veno-arterial extracorporeal membrane oxygenation; VSD, ventricular septal defect
Furthermore, a microaxial pump (Impella, Abiomed, Danvers, MA, USA) device has been suggested as a stand-alone therapy or as a venting method in conjunction with other MCS devices (e.g. ECPELLA: VA-ECMO + microaxial pump), depending on the patient's clinical status and heart failure severity (Figure 6). The microaxial pump provides transvalvular ventricular unloading, decreases PCWP, and increases CO. In patients with large VSD and with increased RV pressures, microaxial pump use is cautioned, as it may cause shunt inversion, namely right-to-left shunt, pushing deoxygenated blood towards the systemic circulation.48,49
A left-atrial-to-systemic MCS (TandemHeart; Livanova, Boston, MA, USA) may be appropriate as an option in patients with MI-VSD.44 However, as for the microaxial pump, preload to the device relies on RV systolic function. Left atrial-to-systemic MCS hemodynamically supports the patient similarly to VA-ECMO, causing an LV afterload increase, but avoids LV dilatation and reduces PCWP by aspirating blood from the left atrium by a transseptal cannula (Figure 6). Hemodynamic effects of IABP, a microaxial pump and a left-atrial-to-systemic MCS appear favourable in the context of MI-VSD (see Supplementary data online, Table S1).
Peripheral VA-ECMO increases afterload and may worsen left-to-right shunting (Figure 6).44,50 Mechanical venting may be appropriate in case of LV distension, pulmonary congestion, non-opening of the aortic valve leading to non-pulsatile arterial flow, or intracavitary blood stasis. There are several strategies of venting [e.g. microaxial pump, IABP, left atrial-to-systemic MCS, atrial septostomy (to lower intracavitary pressures and prevent blood stasis in the left atrium and left ventricle), or other surgical venting strategies (vent insertion into the left atrium or left ventricle)]. However, adequate comparative data are lacking and therefore, the optimal method of venting still remains to be defined.6,46,47
The proper consideration of MCS application time and strategy is critical and aims to delay the time of definitive closure, while maintaining end-organ perfusion, to enhance peri-procedural success rate, reduce the postoperative recurrence rate by unloading the left ventricle for the first days after the repair, and to provide a prolonged support during the vulnerable early post-operative phase. MCS systems may also provide a protected transfer of the patient to more specialized centres.6
MCS therapy, however, has major limitations. Severe bleeding, vascular complications, limb ischemia, thrombotic complications, including embolism and LV thrombosis, the risks associated with the need for antithrombotic treatment, especially in CS with frequently associated coagulopathy, as well as haemolytic anaemia and thrombocytopenia secondary to blood trauma remain major concerns.40,51
The optimal use of MCS in MI-VSD is an area of active research, and future studies may help to refine patient selection criteria, guide device selection, and determine the optimal timing and duration of MCS support.
Procedural timing, risk stratification and patient selection for therapeutic interventions
Timing: immediate vs. deferred vs. late treatment
There is ongoing debate regarding the optimal closure timing given the absence of any RCTs and the bias associated with analysis of observational data. Nevertheless, the most recent North American STEMI guidelines recommend emergency surgical repair in all patients, irrespective of hemodynamic presentation.52 The 2023 European acute coronary syndrome guidelines, on the other hand, recommend a more nuanced approach: prompt surgery for patients with refractory shock or persistent RV dysfunction, and a delayed approach in the remaining patients, if possible beyond day 7 after diagnosis, and if necessary bridged with MCS.8
Several registry analyses suggested higher mortality rates in patients undergoing early surgical MI-VSD closure, with the highest mortality rates observed within the first 24 h.10,19,53–55 The relevance of the time to repair has been shown by several studies: a surgical approach within 7 days from MI-VSD occurrence carries an operative mortality between 40% and 90%, which declines to 10% and 40% after 7 days.14,15 These findings are skewed due to survival and observational bias, with the highest-risk patients dying before surgery. The biological rationale of delaying closure is based on the assumption that scarring of the infarcted tissue may facilitate patch suturing.
In stable patients, optimal VSD closure timing needs to balance the risks of subsequent end-organ failure and/or sudden hemodynamic deterioration with the advantage of allowing tissue scarring, which might lead to better surgical results.15,56 In unstable patients, the benefits of preventing or reversing CS and multiorgan failure need to be balanced against the surgical risk of operating into freshly ischemic myocardium. Likewise, timing also plays a role in interventional repair. However, for interventional repair, prevention or reversal of CS is the main treatment goal when considering the optimal timing of the procedure. Based on current evidence and experience, an approach that integrates the patients’ hemodynamic status and VSD characteristics seems reasonable (Figure 7).
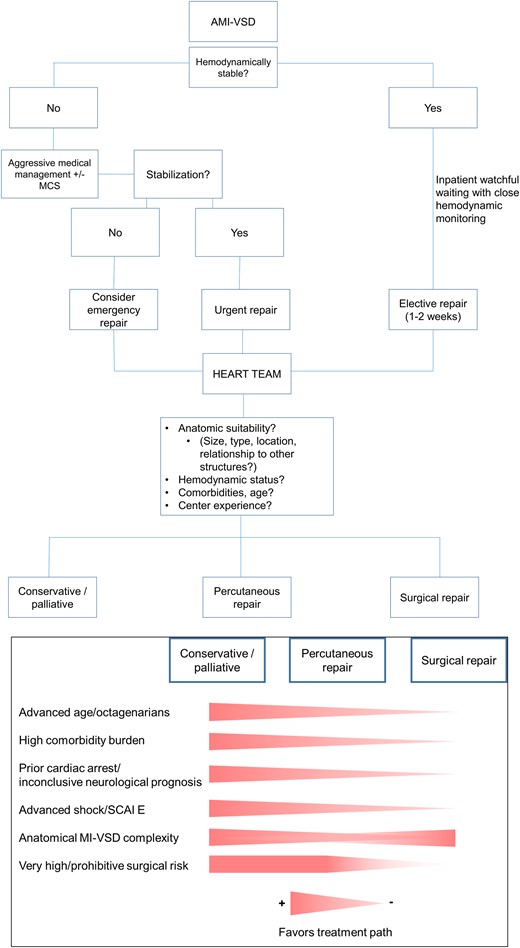
Hemodynamically stable patients with rather small MI-VSDs might qualify for watchful waiting with close hemodynamic monitoring until an elective repair after at least 1–2 weeks of medical therapy. Patients with hemodynamic instability and/or large MI-VSDs with a consecutively high left-to-right shunt require aggressive pharmacologic therapy and possibly MCS treatment. If responsive to treatment, closure may be performed delayed, urgently within the first few days or as an emergency procedure in cases of refractory CS.
Technique: surgical vs. interventional
No head-to-head RCTs comparing surgical with interventional approaches for MI-VSD treatment exist and registry-based comparisons are scarce. Mortality rates of patients undergoing surgical and interventional repair reported in registries and case series are comparable with approximately 45%.13,14,57
The largest head-to-head analysis to date is a retrospective observational study from the UK, including 16 centres performing both percutaneous and surgical closure.34 Both groups (total n = 362) were treated on average 9 days after AMI, with no differences in long-term mortality (61% vs. 54%). In-hospital mortality was lower in the surgical group (44% vs. 55%), despite the fact that patients more often presented in CS compared with patients in the interventional group. However, patient age was significantly higher in the interventional group. Low post-discharge mortality rates suggest durability of both treatment options34 in the context of historically reported conservative management mortality rates exceeding 90%.7,9 Presence of smaller defects, older age and lower overall center procedural volume were associated with the choice of an interventional procedure. Unreported reasoning for the choice of approach and selection bias represent caveats in the analysis of such registry-based data.
Given comparable outcomes in registries and the lack of RCTs, dedicated centre- and regional MI-VSD pathways and heart teams, including experienced physicians in MI-VSD care, should be central for decision-making. Factors to consider for the choice of the therapeutic approach include the patient’s hemodynamic status, the size, type and location of the defect including its interference with other structures as well as the centre experience with either approach (Figure 7). Importantly, surgical and interventional techniques may be applied in concert, as residual shunt remains a concern with both strategies. In the UK registry, in the surgical cohort, 1% of patients required a secondary surgical repair and 7% underwent a subsequent interventional closure. Following an initial interventional strategy, 15% of patients needed subsequent surgical closure and 6% underwent an additional interventional procedure.34
Localization: differential treatment per defect localization necessary?
Several factors should affect the choice of repair technique. Very large defects, anatomical relationship with and involvement of other cardiac structures such as valves and presence of multiple, significant defects favour a surgical approach. Factors favouring a percutaneous approach include inoperability, previous surgical failure and interventional expertise. Patient preferences should be considered as well.
(Concomitant) revascularization strategy
The indication for an invasive coronary angiography should be guided by current clinical guidelines for acute coronary syndromes.8 The majority of patients presenting with STEMI within the first 12 h after symptom onset will undergo emergent coronary angiography and revascularization per current clinical practice guidelines.8,52 In patients presenting 48 h or more after symptom onset, operators should abstain from percutaneous coronary intervention (PCI) of totally occluded vessels. In NSTEMI, in the absence of ongoing ischemia, PCI should only be performed after careful evaluation of the risks and benefits. Before proceeding to PCI in acute coronary syndrome, special attention should be paid to any hint at a MI-VSD. This is of special importance in patients with MI-VSD, as the associated use of P2Y12 inhibitors may preclude or delay a surgical strategy because of possible significant bleeding complications. Any suspicion of a mechanical complication of AMI should lead to prompt evaluation, especially prior to proceeding to PCI. In a UK series on percutaneous VSD repair, approximately half of all patients did not undergo reperfusion prior to repair.57 Interestingly, this group had poorer outcomes. In a larger, more contemporary UK registry analysis including patients treated with both a surgical and a percutaneous approach, PCI of the infarct-related artery was associated with increased mortality.34
The impact of coronary revascularization in addition to surgical MI-VSD repair remains unclear.58 Despite the fact that no RCT exists between MI-VSD repair and coronary artery bypass grafting (CABG) of the infarct-related artery vs. VSD closure alone, the 2021 ACC/AHA/SCAI guidelines recommend infarct-related artery surgical revascularization to improve survival.59 However, this has to be weighed against the risk of reperfusion injury and suture entrapment by the ventriculotomy closure.60–63 On the other hand, a benefit of revascularization of non-infarct-related coronary arteries to avoid further deterioration of the LV function and for long-term outcome has been suggested.64–69 Nevertheless, a recent meta-analysis, showed no difference in early or late mortality between patients submitted to CABG or not.58
Overall, decisions on revascularization should follow current clinical practice guidelines on revascularization in AMI.8,52
Risk prediction and identification of futility
Large registry-based analyses demonstrated mortality rates of around 45% in patients undergoing surgical or percutaneous VSD-AMI closure. Predictors of adverse outcomes that were associated with mortality are older age, presence of CS, renal insufficiency, number of vessels with coronary artery disease, elevated disease severity scores and surgical risk scores.64,70,71 Specifically in the recent UK registry,34 the presence of CS, percutaneous approach and number of vessels with coronary artery disease were independently associated with long-term mortality. In a large Japanese registry of 1397 patients undergoing surgical MI-VSD repair, older age, emergency/salvage cases, preoperative shock, severely compromised ejection fraction, renal failure, and three-vessel disease were independent predictors of perioperative death.71
In such patients, however, especially those with CS, repair remains the only causative treatment. As shown in an analysis of the GUSTO-I trial, conservative treatment is in fact futile, with an observed 94% mortality rate.7
Clinical decision-making should integrate predictors of high mortality. Patient-based predictors [advanced age (octogenarians), high comorbidity burden], shock-based predictors (history of cardiac arrest, rhythm instability, lactate levels, multiorgan failure), and cardiac/VSD-based predictors (severely compromised LV and/or RV function, large defect) may independently and in conjunction with each other serve as predictors of futility.
The development of a futility score for patients in CS with or without MI-VSD would be helpful in reliably identifying patients for a palliative approach.72 Until then, clinical experience should guide decision-making based on the dismal outcomes in patients with a conservative approach.
Role of the heart team in MI-VSD
Treatment algorithm
Recent investigations have shown that more than 50% of MI-VSD patients are admitted with CS, and almost 9% in or after cardiac arrest.34,73 Involvement of a dedicated Shock Team is, therefore, of importance and should include a multidisciplinary group with expertise in the management of refractory CS and should ideally include a cardiovascular intensive care physician, an interventional cardiologist with experience in structural heart disease, a cardiovascular imaging specialist, a heart failure cardiologist, and a cardiac surgeon with experience in structural heart disease.74 Palliative care professionals should be included on top, as an integrated part of the team.6 A palliative approach might be considered over the choice of further aggressive and resource-intensive treatment, making the patient and family part of the strategy and decision-making process, particularly in cases with low survival probability.75
The Shock Team should assess the signs of hemodynamic compromise and propose therapeutic options to control it.6,74 Anatomical and functional VSD features, immediate application of appropriate pharmacological treatment, and the need and type of MCS are the key parts of initial decision-making. Further evaluation timepoints should be defined leaving space for upgrading or modifications of the therapeutic strategy according to the clinical course.74 Positioning of a pulmonary artery catheter might allow better hemodynamic monitoring of the provided management, in association with other multimodal imaging techniques.6 Another step accounts for the type of procedure to be applied. Surgical VSD-closure is still considered by many as first-line therapy. However, risk factors as well as intraoperative and perioperative strategies should be carefully considered by the Shock Team. In the presence of very high or prohibitive operative risk, a multimodal imaging should be established, if the clinical condition allows, and the anatomical details of the VSD should be assessed to consider a percutaneous approach.76
In case of surgery, the repair technique should be discussed and agreed. In case of persistent hemodynamic instability despite pharmacological and IABP-treatment, a bridge-to-surgery strategy with upgraded MCS has shown promising results.77,78 If surgery or interventional closure are considered, the possibility of sudden death during the procedure should be discussed with the patient and the patient’s family.
More advanced therapies, like heart transplantation or durable ventricular assist device, obviously linked to the actual access to such therapies in a short time, might be considered as an alternative strategy for suitable candidates.
Finally, in case of extremely complex anatomical conditions precluding surgical or percutaneous closure in a local setting as well as unavailability of local resources for more advanced therapy, a protected (with temporary MCS) patient transfer to a centre with these facilities should be considered. In case of no transfer option and exclusion of surgical or percutaneous procedure due to futility, palliative care should be discussed by the Shock Team with the patient’s family. A treatment algorithm is provided in Figure 7.
Evidence gaps and scientific agenda
Call for a European Central MI-VSD registry
As outlined above, evidence is limited, and even observational data are limited by mainly single-centre experiences and/or retrospective design if larger multicenter datasets are available. Moreover, the majority of registries represent a highly selected population in whom interventional or surgical repair was attempted. Nearly no contemporary data are available, including all patients with VSD, encompassing also those with conservative approaches or those dying because of a deferred intended closure.
This calls for comprehensive national, European, or even worldwide central MI-VSD prospective registries, including all patients and providing more granular data to describe current treatment strategies and outcome for this rare but devastating disease. Based on this, scores to assess mortality risk or even futility may be generated which will guide treatment decisions.
Potential for RCTs in MI-VSD?
Based on such a multicenter European or even global initiative, RCTs should be initiated to address the multiple open questions. Table 1 provides an overview of gaps in evidence and possible RCT designs (see also Graphical Abstract). Such RCTs, however, will only be possible based on huge joined academic efforts and by combined funding efforts such as the European Horizon 2020 program or under other international joined forces like the Global Cardiovascular Research Funder Forum (GCRFF). Since no single national initiative will be able to randomize the required number of patients, if a proper sample size calculation is performed and hard endpoints are chosen, only a European or worldwide initiative may be successful.
Gaps of evidence in myocardial infarction ventricular septal defect (MI-VSD) care and suggested randomized controlled trials (RCTs) to address these
| Gaps in MI-VSD Care . | Needed RCTs . |
|---|---|
| Risk Prediction Modelling | |
| Whether risk stratification of MI-VSD patients based on multivariable risk prediction models and also defining futility improves clinical outcomes remains unclear. | Patients randomized to treatment algorithms based on scores calculated at point of care or to usual treatment. |
| Treatment Strategies | |
| Timing with or without MCS | |
| Timing of surgical closure, i.e. immediate vs. delayed, in stable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) surgical repair. |
| Timing of surgical closure, i.e. immediate vs. delayed, in unstable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) surgical repair including advanced MCS for stabilization. |
| Timing of interventional closure, i.e. immediate vs. delayed, in stable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) interventional repair. |
| Timing of interventional closure, i.e. immediate vs. delayed, in unstable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) interventional repair including advanced MCS for stabilization. |
| MCS | |
| Should interventional closure in stable VSD patients be performed with prophylactic MCS. | Randomized trial of interventional closure with or without prophylactic MCS. |
| Surgery or Interventional Closure | |
| Whether surgery or interventional closure leads to better clinical outcome remains undetermined. | Randomized trial of surgical vs. interventional closure after Heart Team eligibility assessment. |
| Revascularization | |
| Whether revascularization of the infarct-related artery improves outcome is unclear. | Randomized trial of infarct-related artery revascularization in addition to surgical or interventional closure. |
| Whether additional complete revascularization to MI-VSD closure improves outcome is unclear. | Randomized trial of surgical or interventional closure with or without complete revascularization. |
| Gaps in MI-VSD Care . | Needed RCTs . |
|---|---|
| Risk Prediction Modelling | |
| Whether risk stratification of MI-VSD patients based on multivariable risk prediction models and also defining futility improves clinical outcomes remains unclear. | Patients randomized to treatment algorithms based on scores calculated at point of care or to usual treatment. |
| Treatment Strategies | |
| Timing with or without MCS | |
| Timing of surgical closure, i.e. immediate vs. delayed, in stable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) surgical repair. |
| Timing of surgical closure, i.e. immediate vs. delayed, in unstable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) surgical repair including advanced MCS for stabilization. |
| Timing of interventional closure, i.e. immediate vs. delayed, in stable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) interventional repair. |
| Timing of interventional closure, i.e. immediate vs. delayed, in unstable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) interventional repair including advanced MCS for stabilization. |
| MCS | |
| Should interventional closure in stable VSD patients be performed with prophylactic MCS. | Randomized trial of interventional closure with or without prophylactic MCS. |
| Surgery or Interventional Closure | |
| Whether surgery or interventional closure leads to better clinical outcome remains undetermined. | Randomized trial of surgical vs. interventional closure after Heart Team eligibility assessment. |
| Revascularization | |
| Whether revascularization of the infarct-related artery improves outcome is unclear. | Randomized trial of infarct-related artery revascularization in addition to surgical or interventional closure. |
| Whether additional complete revascularization to MI-VSD closure improves outcome is unclear. | Randomized trial of surgical or interventional closure with or without complete revascularization. |
MCS, mechanical circulatory support; MI-VSD, myocardial infarction ventricular septal defect; RCT, randomized controlled trial.
Gaps of evidence in myocardial infarction ventricular septal defect (MI-VSD) care and suggested randomized controlled trials (RCTs) to address these
| Gaps in MI-VSD Care . | Needed RCTs . |
|---|---|
| Risk Prediction Modelling | |
| Whether risk stratification of MI-VSD patients based on multivariable risk prediction models and also defining futility improves clinical outcomes remains unclear. | Patients randomized to treatment algorithms based on scores calculated at point of care or to usual treatment. |
| Treatment Strategies | |
| Timing with or without MCS | |
| Timing of surgical closure, i.e. immediate vs. delayed, in stable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) surgical repair. |
| Timing of surgical closure, i.e. immediate vs. delayed, in unstable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) surgical repair including advanced MCS for stabilization. |
| Timing of interventional closure, i.e. immediate vs. delayed, in stable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) interventional repair. |
| Timing of interventional closure, i.e. immediate vs. delayed, in unstable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) interventional repair including advanced MCS for stabilization. |
| MCS | |
| Should interventional closure in stable VSD patients be performed with prophylactic MCS. | Randomized trial of interventional closure with or without prophylactic MCS. |
| Surgery or Interventional Closure | |
| Whether surgery or interventional closure leads to better clinical outcome remains undetermined. | Randomized trial of surgical vs. interventional closure after Heart Team eligibility assessment. |
| Revascularization | |
| Whether revascularization of the infarct-related artery improves outcome is unclear. | Randomized trial of infarct-related artery revascularization in addition to surgical or interventional closure. |
| Whether additional complete revascularization to MI-VSD closure improves outcome is unclear. | Randomized trial of surgical or interventional closure with or without complete revascularization. |
| Gaps in MI-VSD Care . | Needed RCTs . |
|---|---|
| Risk Prediction Modelling | |
| Whether risk stratification of MI-VSD patients based on multivariable risk prediction models and also defining futility improves clinical outcomes remains unclear. | Patients randomized to treatment algorithms based on scores calculated at point of care or to usual treatment. |
| Treatment Strategies | |
| Timing with or without MCS | |
| Timing of surgical closure, i.e. immediate vs. delayed, in stable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) surgical repair. |
| Timing of surgical closure, i.e. immediate vs. delayed, in unstable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) surgical repair including advanced MCS for stabilization. |
| Timing of interventional closure, i.e. immediate vs. delayed, in stable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) interventional repair. |
| Timing of interventional closure, i.e. immediate vs. delayed, in unstable MI-VSD patients is not clear. | Dedicated randomized trials of immediate vs. delayed (e.g. 2–3 weeks after VSD occurrence) interventional repair including advanced MCS for stabilization. |
| MCS | |
| Should interventional closure in stable VSD patients be performed with prophylactic MCS. | Randomized trial of interventional closure with or without prophylactic MCS. |
| Surgery or Interventional Closure | |
| Whether surgery or interventional closure leads to better clinical outcome remains undetermined. | Randomized trial of surgical vs. interventional closure after Heart Team eligibility assessment. |
| Revascularization | |
| Whether revascularization of the infarct-related artery improves outcome is unclear. | Randomized trial of infarct-related artery revascularization in addition to surgical or interventional closure. |
| Whether additional complete revascularization to MI-VSD closure improves outcome is unclear. | Randomized trial of surgical or interventional closure with or without complete revascularization. |
MCS, mechanical circulatory support; MI-VSD, myocardial infarction ventricular septal defect; RCT, randomized controlled trial.
However, the lack of any RCT evidence in this disease with very high mortality calls for such a global MI-VSD initiative to improve the still dismal prognosis.
Conclusion
The resource-intense care of patients with MI-VSD remains a major challenge and data to guide clinical decision-making is scarce. In the complex hemodynamic and ethical environment of MI-VSD, necessary and indicated highly invasive strategies have to be weighed against the associated risks and the potential of futility despite aggressive therapy. MCS may have a role to temporarily bypass some of the hemodynamic alterations of MI-VSD as a bridge to definitive repair as the only viable treatment option. However, MI-VSD-associated morbidity and mortality remains very high. Future systematic scientific investigations into MI-VSD may provide enhanced treatment recommendations.
Supplementary Data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
Nothing to declare.
Data Availability
No data were generated or analysed for or in support of this paper.
Funding
Nothing to declare.
References
Author notes
Association for Acute CardioVascular Care (ACVC).
European Society of Cardiology Working Group on Cardiovascular Surgery.
European Association of Percutaneous Cardiovascular Interventions (EAPCI).



