-
Views
-
Cite
Cite
Osung Kwon, Jong-Hwa Ahn, Jin-Sin Koh, Yongwhi Park, Seok Jae Hwang, Udaya S Tantry, Paul A Gurbel, Jin-Yong Hwang, Young-Hoon Jeong, Platelet-fibrin clot strength and platelet reactivity predicting cardiovascular events after percutaneous coronary interventions, European Heart Journal, Volume 45, Issue 25, 1 July 2024, Pages 2217–2231, https://doi.org/10.1093/eurheartj/ehae296
Close - Share Icon Share
Abstract
Platelet-fibrin clot strength (PFCS) is linked to major adverse cardiovascular event (MACE) risk. However, the association between PFCS and platelet reactivity and their prognostic implication remains uncertain in patients undergoing percutaneous coronary intervention (PCI).
In PCI-treated patients (n = 2512) from registry data from January 2010 to November 2018 in South Korea, PFCS using thromboelastography and platelet reactivity using VerifyNow were measured. High PFCS (PFCSHigh) was defined as thromboelastography maximal amplitude ≥ 68 mm, and high platelet reactivity (HPR) was defined as >208 P2Y12 reaction units. Patients were stratified into four groups according to maximal amplitude and P2Y12 reaction unit levels: (i) normal platelet reactivity (NPR)-PFCSNormal (31.8%), (ii) HPR-PFCSNormal (29.0%), (iii) NPR-PFCSHigh (18.1%), and (iv) HPR-PFCSHigh (21.1%). Major adverse cardiovascular event (all-cause death, myocardial infarction, or stroke) and major bleeding were followed up to 4 years.
High platelet reactivity and PFCSHigh showed an additive effect for clinical outcomes (log-rank test, P < .001). Individuals with NPR-PFCSNormal, NPR-PFCSHigh, HPR-PFCSNormal, and HPR-PFCSHigh demonstrated MACE incidences of 7.5%, 12.6%, 13.4%, and 19.3%, respectively. The HPR-PFCSHigh group showed significantly higher risks of MACE compared with the NPR-PFCSNormal group [adjusted hazard ratio (HRadj) 1.89; 95% confidence interval (CI) 1.23–2.91; P = .004] and the HPR-PFCSNormal group (HRadj 1.60; 95% CI 1.12–2.27; P = .009). Similar results were observed for all-cause death. Compared with HPR-PFCSNormal phenotype, NPR-PFCSNormal phenotype was associated with a higher risk of major bleeding (HRadj 3.12; 95% CI 1.30–7.69; P = .010).
In PCI patients, PFCS and platelet reactivity demonstrated important relationships in predicting clinical prognosis. Their combined assessment may enhance post-PCI risk stratification for personalized antithrombotic therapy.
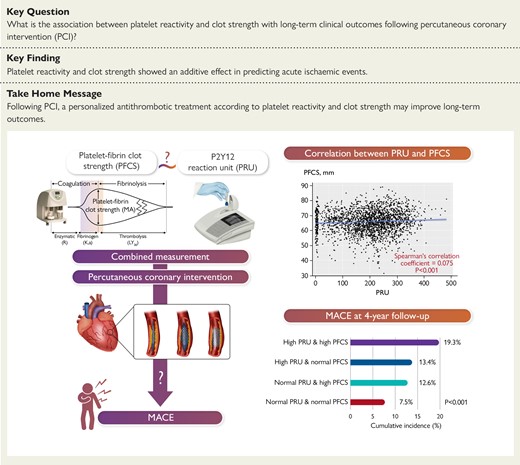
Major adverse cardiovascular events at 4-year follow-up after PCI were stratified by PRU and PFCS. In PCI-treated patients, ‘high PFCS phenotype’ and HPR demonstrated an additive effect for MACE occurrence, and patients with both phenotypes showed the highest risk of MACE. Their combined assessment would enhance post-PCI risk stratification for personalized antithrombotic therapy. HPR, high platelet reactivity; MA, maximal amplitude; MACE, major adverse cardiovascular event; NPR, normal platelet reactivity; PCI, percutaneous coronary intervention; PFCS, platelet-fibrin clot strength; PRU, P2Y12 reaction unit.





