-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, The grey areas in anticoagulation for stroke prevention and a focus on chronic stress in carotid atherosclerosis, European Heart Journal, Volume 45, Issue 19, 14 May 2024, Pages 1687–1691, https://doi.org/10.1093/eurheartj/ehae285
Close - Share Icon Share
 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
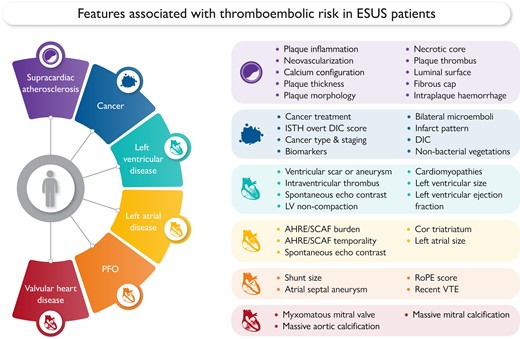
This Focus Issue on thrombosis and antithrombotic treatment and on vascular biology and medicine contains the Special Article entitled ‘Embolic strokes of undetermined source: a multidisciplinary consensus statement of the ESC Council on Stroke, the European Association of Cardiovascular Imaging and the European Heart Rhythm Association of the ESC’ by George Ntaios from the University of Thessaly in Greece.1 Stroke is an invalidating disease with multiple causes.2–8 The authors point out that one in six ischaemic stroke patients has an embolic stroke of undetermined source (ESUS), defined as stroke with unclear aetiology despite recommended diagnostic evaluation. The overall cardiovascular risk of ESUS is high and it is important to optimize strategies to prevent recurrent stroke and other cardiovascular events. The aim of clinicians when confronted with a patient not only with ESUS but with any other medical condition of unclear aetiology is to identify the specific cause in order to optimize secondary prevention. However, in patients with ESUS this may be challenging, as multiple potential thromboembolic sources frequently coexist. Therefore, rather than trying to identify a single embolic source, it is important to assess the overall thromboembolic risk of the patient through the identification of the individual risk linked to each potential embolic source, regardless if presumed causally associated to stroke. In this paper, a multidisciplinary panel of clinicians/researchers from different specialties (cardiology, internal medicine, neurology, radiology) proposes a comprehensive multidimensional assessment of the overall thromboembolic risk in ESUS patients through the assessment of the individual risk associated to each potential embolic source (Figure 1).
In a State of the Art Review article entitled ‘Aspirin hypersensitivity: a practical guide for cardiologists’, Raffaele De Caterina from the University of Pisa School of Medicine and Surgery in Italy and colleagues note that aspirin has been known for a long time.9 Aspirin currently remains a cornerstone of antithrombotic therapy in cardiovascular disease.10,11 In patients with either acute or chronic coronary syndromes undergoing percutaneous coronary intervention, aspirin is mandatory in a dual antiplatelet therapy regimen for prevention of stent thrombosis and/or new ischaemic events. Aspirin is also currently a first-option antithrombotic therapy after an aortic prosthetic valve replacement and is occasionally required in addition to oral anticoagulants after implantation of a mechanical valve. Presumed or demonstrated aspirin hypersensitivity is the main clinical problem, limiting the use of a life-saving medication. In the general population, aspirin hypersensitivity has a prevalence of 0.5% to 2.4% and has a plethora of clinical presentations, ranging from aspirin-exacerbated respiratory disease to anaphylaxis. Although infrequent, when encountered in clinical practice aspirin hypersensitivity poses a clinical dilemma for cardiologists, which should never be trivialized, thus avoiding—as much as possible—omission of the drug. In this contribution, the epidemiology of aspirin hypersensitivity is reviewed, providing an outline of pathophysiological mechanisms and clinical presentations, as well as management options, starting from a characterization of true aspirin allergy—in contrast to intolerance—to suggestion of desensitization protocols.
These contributions are followed by two Viewpoints from our ‘Year in Cardiovascular Medicine’ series. My goal in commissioning this series again for 2023 was to get an overview of some of the most impactful papers in various areas of expertise. In ‘The year in cardiovascular medicine 2023: the top 10 papers in thrombosis and antithrombotic treatment’, EHJ editors Felicita Andreotti, Michelle O’Donoghue and Jurriën Ten Berg point out that preventing thrombosis while minimizing bleeding is paramount in antithrombotic therapy. Recent progress this last year has brought this goal closer. This contribution highlights recent pivotal papers on antithrombotic strategies.12 In ‘The year in cardiovascular medicine 2023: the top 10 papers in arrhythmias’, EHJ editors Harry Crijns, Pier Lambiase and Prashantan Sanders point out that the last year was marked by fascinating new discoveries, particularly in the field of atrial fibrillation (AF) and long QT syndrome (LQTS).13 These advanced our understanding of arrhythmias. The authors anticipate paradigm shifts in screening for AF, treatment of subclinical AF and LQTS.
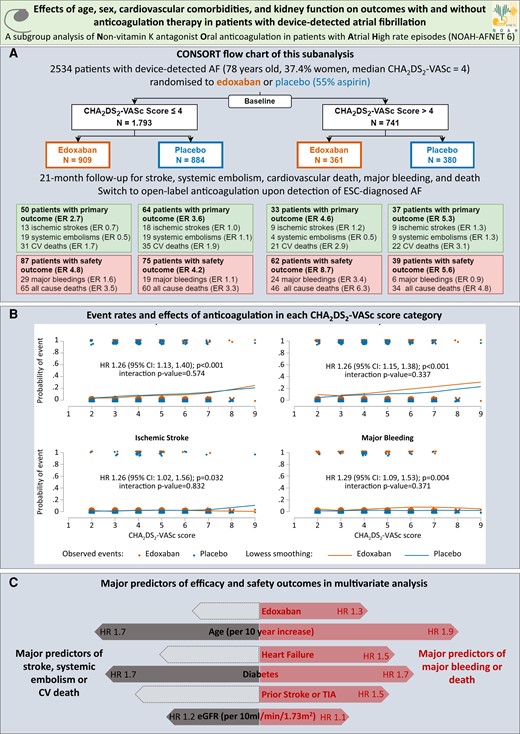
(A) CONSORT flow chart of pre-specified secondary analysis of the NOAH-AFNET 6 trial. Displayed are the analysis population, the number of patients experiencing a primary or safety outcome and the event rate for each outcome in each group. (B) Stroke, systemic embolism or cardiovascular death (primary outcome), major bleeding or death (safety outcome), ischaemic stroke and major bleeding event rate estimates per CHA2DS2-VASc score and treatment group (edoxaban orange on the left, placebo blue on the right). The LOWESS (locally weighted scatterplot smoothing) curves show the dependence of the probability of an event on the CHA2DS2-VASc score. Each dot represents a patient. Patients with events are shown at the top and patients without events are shown at the bottom. (C) Forest plots of the major predictors of efficacy (left) and safety (right) outcomes in the entire study population (n = 2534). Grey shaded arrows indicate efficacy predictors with P-values > .05. Orange curves show LOWESS-estimated event rates with edoxaban, blue curves show LOWESS-estimated event rates without anticoagulation. AF, atrial fibrillation; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ER, event rate per 100 patient-years follow-up; ESC, European Society of Cardiology; HR, hazard ratio; TIA, transient ischaemic attack14
In a Rapid Communications article entitled ‘Oral anticoagulation in device-detected atrial fibrillation: effects of age, sex, cardiovascular comorbidities, and kidney function on outcomes in the NOAH-AFNET 6 trial’, Gregory Lip from the University of Liverpool in the United Kingdom and colleagues remind us that implanted pacemakers, defibrillators and loop recorders detect short and rare episodes of device-detected atrial fibrillation (DDAF, previously also called atrial high-rate episodes or subclinical AF) in ∼30% of patients.14 Whereas the utilization of anticoagulant is well established in patients with ECG-documented AF,4,8,15 DDAF without ECG-documented AF can lead to consideration of oral anticoagulation in clinical practice, especially in older patients with multiple stroke risk factors and/or very long DDAF episodes, largely based on observational data. Two recent controlled trials, NOAH-AFNET 6 and ARTESiA, observed a low rate of ischaemic stroke without anticoagulation (1.1%–1.2%/patient-year) in patients with DDAF and stroke risk factors, including in patients with very long DDAF episodes in NOAH-AFNET 6.16,17 Current guidelines leave the decision to anticoagulate to clinical judgement, balancing the expected stroke risk, typically estimated by using stroke risk scores developed in patients with ECG-documented AF, and the stroke risk reduction induced by anticoagulation, with the increase in bleeding associated with anticoagulation therapy. This pre-specified subgroup analysis of the NOAH-AFNET 6 trial data set compares outcomes and the effect of oral anticoagulation in patients with DDAF without ECG-documented AF and a CHA2DS2-VASc score > 4 to those with fewer CHA2DS2-VASc factors. In the subgroup of patients with a CHA2DS2-VASc score > 4, stroke, systemic embolism or cardiovascular death occurred in 33/361 patients with anticoagulation and in 37/380 patients without anticoagulation (HR 0.88). The rate of stroke was low with and without anticoagulation (1.2–1.3/100 patient-years). In the total population, efficacy and safety outcome rates increased with increasing CHA2DS2-VASc scores without treatment interaction (Figure 2).
The authors conclude that taking into account the limited statistical power of any subanalysis of a large controlled trial, these results highlight the ambiguous effects of anticoagulation in patients with DDAF, including in patients with multiple comorbidities and with long DDAF episodes. The findings call for new methods to identify patients with DDAF at high risk of stroke who might benefit from anticoagulation.
Peripheral artery disease (PAD) is a prevalent and high-risk clinical manifestation of atherothrombosis.10,18 Few studies have compared arm and ankle blood pressures (BPs) with regard to PAD and mortality. In a Clinical Research article entitled ‘Arm and ankle blood pressure indices, and peripheral artery disease, and mortality: a cohort study’, Kamel Mohammedi from McMaster University in Hamilton, ON (Canada) and colleagues assessed these relationships using data from three large prospective clinical trials.19 Baseline BP indices included arm systolic BP (SBP), diastolic BP (DBP), pulse pressure (arm SBP minus DBP), ankle SBP, ankle–brachial index (ABI, ankle SBP divided by arm SBP) and ankle–pulse pressure difference (APPD, ankle SBP minus arm pulse pressure). These measurements were categorized into four groups using quartiles. The outcomes were PAD (the first occurrence of either peripheral revascularization or lower-limb amputation for vascular disease), the composite of PAD or death, and all-cause death. Among about 41 000 participants without baseline PAD (age 66 years, men %, diabetes 50.2%) from 53 countries, 2.6% developed PAD, and 12.2% died during 5 years of follow-up. Incident PAD progressively rose with higher arm BP indices. The strongest relationships, however, were noted for ankle BP indices. Compared with people whose ankle BP indices were in the highest fourth, adjusted hazard ratios for each lower fourth were 1.64, 2.59 and 4.23 for ankle SBP; 1.19, 1.66 and 3.34 for ABI; and 1.41, 2.04 and 3.63 for APPD. Similar patterns were observed for mortality. Ankle BP indices provided the highest c-statistics and classification indices in predicting future PAD beyond established risk factors.
The authors conclude that ankle BP indices including the ankle SBP and the APPD best predicted PAD and mortality. The contribution is accompanied by an Editorial by Mounica Yanamandala and Marie Denise Gerhard-Herman from Brigham and Women's Hospital and Harvard Medical School in Boston, MA (USA) and Guillaume Goudot from the Université Paris Cité, Vascular Medicine Department in France.20 The authors note that going forward, we must consider that much of the literature since the 1950s and our understanding of the epidemiology, management standards and morbidity and mortality associated with PAD is based on traditional assessment of ABI. Ankle SBP and APPD do not solve the challenge of diagnosing PAD in patients with stiff vessels, such as in diabetics and/or chronic kidney disease (CKD). Ultimately, newer functional tests that provide information about perfusion to the muscle such as positron emission tomography or magnetic resonance are needed to overcome challenges with PAD diagnosis and management, especially in those with diabetes or CKD.
Chronic stress associates with cardiovascular disease, but neural circuits underlying this association remain incompletely defined. In a Clinical Research article entitled ‘Cortico-limbic interactions and carotid atherosclerotic burden during chronic stress exposure’, David O'Connor from the Icahn School of Medicine at Mount Sinai New York, in New York (USA) and colleagues used advanced imaging to identify stress-related neural imaging phenotypes associated with atherosclerosis.21 Twenty-seven individuals with posttraumatic stress disorder (PTSD), 45 trauma-exposed controls without PTSD (TC), and 22 healthy controls (HC) underwent 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging (18F-FDG-PET/MRI). Atherosclerotic burden and inflammation were assessed using vascular MRI and 18F-FDG-PET, respectively. Inflammation was assessed using high-sensitivity C-reactive protein (hsCRP) and leukopoietic imaging (18F-FDG-PET uptake in spleen and bone marrow). Stress-associated neural network activity (SNA) was assessed on 18F-FDG-PET as amygdala relative to ventromedial prefrontal cortex (vmPFC) activity. MRI diffusion tensor imaging assessed the axonal integrity (AI) of the uncinate fasciculus (major white matter tract connecting vmPFC and amygdala). PTSD had similar atherosclerotic inflammation to controls but had higher hsCRP, spleen activity and aorta atherosclerotic burden (normalized wall index). PTSD also had higher SNA and lower AI. Across the study cohort, carotid atherosclerotic burden associated positively with SNA (P = 0.001) and negatively with AI (P < 0.001). A high SNA and low AI combination associated with carotid atherosclerotic burden independent of Framingham risk score.
O'Connor et al. conclude that impaired corticolimbic interactions (higher amygdala relative to vmPFC activity or disruption of their intercommunication) associate with carotid atherosclerotic burden. These findings refine the brain–heart connection and serve as basis for larger prospective studies. The manuscript is accompanied by an Editorial by Sarajo Mohanta, Donato Santovito and Christian Weber from the Ludwig-Maximilians-Universität (LMU) in Munich, Germany.22 The authors conclude by noting that the study by Gharios et al. provides important insights into the potential relevance of the corticolimbic inflammatory networks in human atherosclerosis and its association with chronic psychological stress. Although future research is required to delineate the complexity of the neural connections between the brain and the cardiovascular system, future studies may hold the promise to develop novel therapeutic interventions in cardiovascular–brain circuits to treat atherosclerosis beyond current lipid-lowering and anti-inflammatory therapies.
The editors hope that this issue of the European Heart Journal will find the interest of its readers.
Dr. Crea reports speaker fees from Abbott, Amgen, Astra Zeneca, BMS, Chiesi, Daiichi Sankyo, Menarini outside the submitted work.
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.




