-
PDF
- Split View
-
Views
-
Cite
Cite
Miron Sopic, Victoria Stopa, Yvan Devaux, Leveraging epitranscriptomics for cardiovascular disease theranostics, European Heart Journal, Volume 45, Issue 13, 1 April 2024, Pages 1098–1100, https://doi.org/10.1093/eurheartj/ehad852
Close - Share Icon Share
The need for new avenues in cardiovascular diseases management
While advancements in diagnostic and therapeutic protocols have undoubtedly improved the management of cardiovascular diseases (CVD), these conditions continue to exert a substantial global healthcare burden. Acute events, such as myocardial infarction and sudden cardiac arrest, can result in fatalities in a significant number of cases. Furthermore, survivors of these acute events face an elevated risk of major complications, including recurrent incidents, arrhythmias, heart failure, etc.1,2 Currently, the available tools for predicting and reducing adverse outcomes are scarce, especially those concerning the brain–heart axis.3 To face these challenges, there is an urgent and compelling need for the development of innovative prognostic tools capable of providing timely and reliable predictions of potential complications. Predicting outcomes in CVD patients is vital for helping healthcare providers allocate resources more efficiently and tailor treatment strategies to individual patient needs. As researchers explore novel molecular mechanisms that may unveil previously unrecognized pathological contributors, they are also discovering innovative approaches that could improve CVD management. One such approach, known as theranostics, encompasses the concept of using the same molecules for both therapy and diagnostics. Identifying novel dynamic pathophysiological processes that can be modified and monitored may open new doors to applications in theranostics.
Epitranscriptomics—modifying the RNA landscape
Such actionable processes include RNA modifications, englobed into the terms ‘Epitranscriptomics’ or ‘RNA editing’. Epitranscriptomics covers a new concept that aims to investigate how co- and/or post-transcriptional modifications of RNA transcripts (coding and non-coding RNAs) influence the fate and functions of RNAs in development, homeostasis, and disease states.4 These modifications involve the replacement of one base with another, as seen in A-to-I (adenosine to inosine) or C-to-U (cytosine to uridine) editing. Others do not entail base replacements but rather the addition of small groups to the RNA structure, such asm5C (5-hydroxymethylcytosine), m7G (7-methylguanosine), m1A (N1-methyladenosine), and N6-methyladenosine (m6A). As one of the most prevalent RNA modifications in eukaryotes, m6A plays a central role in various biological processes and is under the regulation of multiple enzymatic activities.5 The addition of m6A is orchestrated by an enzyme complex known as the ‘writer,’ composed of key catalytic components like methyltransferase 3 (METTL3) and RNA-binding elements such as methyltransferase 14 (METTL14), along with cofactors like Wilms tumour-associated protein-1 (WATAP) and KIAA1429. Conversely, the ‘erasers’ are a group of enzymes responsible for the removal of methyl groups from adenosine, which includes fat-mass and obesity-associated protein (FTO) and Alpha-ketoglutarate-dependent dioxygenase AlkB Homolog 5 (ALKBH5). Furthermore, the ‘readers’ are a class of proteins with the ability to identify and bind specific m6A modifications on RNA, exemplified by members like YTH domain-containing family protein 1 (YTHDC1, YTHDF1, YTHDF2, and YTHDF3) and Insulin-like growth factor 2 mRNA binding protein 1 (IGF2BP1). These reader proteins exert influence over critical cellular processes such as translation, splicing, and RNA decay.5
Studies have emphasized the dysregulation and functional significance of m6A in the pathophysiology of atherosclerosis, coronary heart diseases, hypertension, heart failure, cardiac hypertrophy, and stroke.5–7 Studies addressing the biomarker potential of m6A and other epitranscriptomics marks are however more scarce. In a proof-of-concept study, blood and cardiac levels of m6A and FTO were dysregulated in infarcted rat hearts and low circulating levels of m6A were associated with the development of heart failure in patients with acute myocardial infarction.7 Whether m6A can be used for heart failure prediction in this context remains to be determined.
RNA modifications for cardiovascular theranostics
The potential for utilizing m6A in theranostic applications for CVD requires in-depth exploration. The dynamic character of m6A modification unveils possibilities for theranostics, where monitoring of m6A dysregulation may allow identifying patients who would mostly benefit from therapeutic actions to restore m6A homeostasis. This potential is further reinforced by the advanced technological capabilities to precisely quantify m6A levels, as well as other epitranscriptomic marks, employing techniques such as liquid chromatography coupled with mass spectrometry. While these methods provide evaluations of total m6A and other RNA modifications, innovative approaches like methylated RNA immunoprecipitation sequencing (MeRIP-Seq) and direct RNA sequencing with Oxford Nanopore Technologies empower us to gain insights into the presence of modifications at a single-nucleotide resolution.8 As a result, these cutting-edge technologies offer significant promise for assessing the biomarker potential of epitranscriptomic marks, with their dynamic nature making them ideally suited for theranostic applications.
Currently, due to the ground-breaking success of mRNA-based COVID-19 vaccines facilitated by the works of 2023 Nobel Prize in Physiology and Medicine awardees Profs. Katalin Karikó and Drew Weissman, the field of RNA therapeutics is experiencing a rapid evolution. The use of RNA interference [utilizing small interfering RNAs (siRNAs) or short hairpin RNAs] and antisense oligonucleotides (including gapmers, agomirs, and antagomirs) is gaining increasing attention for treating various diseases, including CVD. However, the question arises if these approaches can be used to influence m6A RNA modification, and if that could provide beneficial effects. So far, manipulating the m6A machinery of writers and erasers has shown promise in conferring beneficial cardiovascular effects. A landmark paper reported that down-regulating FTO using siRNAs impaired the contractility of isolated cardiomyocytes while FTO overexpression reduced fibrosis and enhanced angiogenesis in mouse models of myocardial infarction.9 These effects were attributed to regulation of m6A by FTO. Additionally, chemical inhibition of FTO has been associated with reduced inflammation and injury in hyperlipidaemia-induced cardiomyopathy.10 Elsewhere, silencing METTL3 with siRNAs has demonstrated attenuation of cardiac fibrosis induced by myocardial infarction.11 However, these interventions lead to alterations in global m6A levels across transcriptome, and thus may lack specificity required for therapeutic use. Targeting specific m6A signatures on distinct RNAs may represent a more precise and effective approach to modulate/restore dysregulated RNA modifications. The advent of clustered regularly interspaced short palindromic repeats (CRISPR) technology, discovered by 2020 Nobel Prize in Chemistry awardees Profs. Emmanuelle Charpentier and Jennifer Doudna, enables going beyond conventional genome editing, i.e. targeting gene-expression control, epigenome editing, single-nucleotide editing, and RNA editing.12 Leveraging CRISPR to modify/restore sequence-specific m6A modifications on particular RNA transcripts holds potential to shift from broad, global changes to precise, sequence-specific RNA modifications, thus paving the way for advancements in RNA therapeutics.
The discovery of blood-based epitranscriptomic biosignatures may enhance our ability to predict disease development and progression accurately. This could facilitate the identification of high-risk patients who might benefit from strategies like CRISPR-based RNA editing. M6A editing, known for its inducible and reversible nature, holds the potential to pave the way for novel therapeutic agents. Ultimately, the capacity to modify crucial epitranscriptomic signatures and monitor their dynamic changes during the recovery period can enable a more personalized, targeted approach integrating therapy and diagnostics into the realm of theranostics. The importance of research in the RNA field has been recognized by two Nobel Prizes in last three years. Continuing collaborative (e.g. transatlantic) efforts may well find novel utility for RNA and epitranscriptomics as theranostics options (Figure 1).
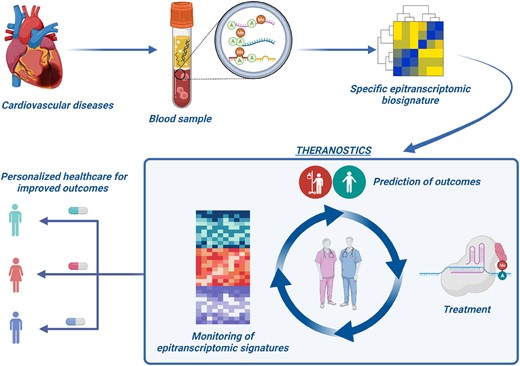
Leveraging epitranscriptomic biosignatures to monitor CVD and design novel therapeutic strategies. A ‘Theranostics’ concept. The figure was created in BioRender
Declarations
Disclosure of Interest
Y.D. holds patents and licencing agreements related to the use of RNAs for diagnostic and therapeutic purposes, and is member of the Scientific Advisory Board of Firalis SA. Other authors have no conflict of interest.
Funding
M.S. is funded by the Horizon Europe (HORIZON-MSCA-2021-PF- MAACS 101064175), the Ministarstvo prosvete, nauke i tehnoloskog razvoja Republike Srbije Ministry of Science, Technological Development and Innovation, Republic of Serbia through a Grant Agreement with the University of Belgrade-Faculty of Pharmacy No: 451-03-47/2023-01/200161. V.S. is supported by a fellowship from the Heart Foundation—Daniel Wagner. Y.D. is funded by the EU Horizon 2020 project COVIRNA (grant agreement # 101016072), the National Research Fund (grants # C14/BM/8225223, C17/BM/11613033 and COVID-19/2020-1/14719577/miRCOVID), the Ministry of Higher Education and Research, and the Heart Foundation-Daniel Wagner of Luxembourg.



