-
PDF
- Split View
-
Views
-
Cite
Cite
M Abuelazm, A M Attia, K Albakri, U Jafar, O Almaadawy, M A Elzeftawy, A Mahmoud, M Ibrahim, B Abdelazeem, Soluble guanylate cyclase in heart failure: A network meta-analysis and subgroup analyses of reduced and preserved ejection fraction, European Heart Journal, Volume 44, Issue Supplement_2, November 2023, ehad655.969, https://doi.org/10.1093/eurheartj/ehad655.969
Close - Share Icon Share
Abstract
Heart failure (HF), either with reduced ejection fraction (HFrEF) or preserved ejection fraction (HFpEF), is a significant healthcare burden, with about one million hospitalizations annually in the United States alone. Soluble guanylate cyclase (sGC) stimulators have been investigated for HF treatment in several randomized controlled trials (RCTs). However, its place in the management guidelines of either HFrEF or HfpEF is still inconclusive.
We aim to evaluate the efficacy and safety of sGC in HF and determine the most effective agent and dosage for the management of either HFrEF or HFpEF.
We conducted a systematic review and network meta-analysis synthesizing RCTs investigating sGC, including vericiguat, riociguat, and praliciguat for HF management, which were retrieved by systematically searching five databases until January 24th, 2023. Dichotomous outcomes were pooled using risk ratio (RR) along with confidence interval (CI). Frequentist random-effects network meta-analysis was conducted via R software using meta and netmeta packages.
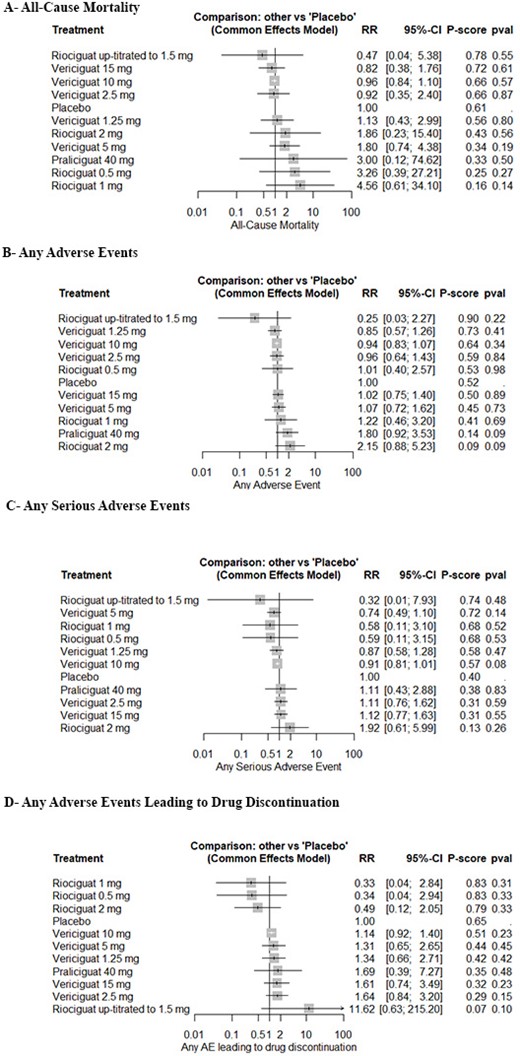
Eight RCTs with a total of 7307 patients were included in our analysis. Four RCTs investigated vericiguat, three investigated riociguat, and another investigated praliciguat. Five RCTs investigated HFpEF patients, and three investigated HFrEF patients. Vericiguat 10 mg significantly reduced the incidence of composite cardiovascular (CVS) mortality and HF hospitalization in HF (RR: 0.88, 95% CI [0.79; 0.98], P = 0.02) and in HFrEF (RR: 0.87, 95% CI [0.78; 0.97], P = 0.02); however, it was not effective in HFpEF (RR: 0.69, 95% CI [0.15; 3.05], P = 0.62). Also, vericiguat 10 mg showed no difference compared to placebo regarding the incidence of all-cause mortality (RR: 0.96, 95% CI [0.84; 1.10], P = 0.57), any adverse events (AEs) (RR: 0.94, 95% CI [0.83; 1.07], P = 0.34), any serious AEs (RR: 0.91, 95% CI [0.81; 1.01], P = 0.08), and any AEs leading to drug discontinuation (RR: 1.14, 95% CI [0.92; 1.40], P = 0.23). However, all other comparisons of vericiguat, riociguat, and praliciguat showed no difference compared to placebo regarding the composite CVS mortality and HF hospitalization, with a similar safety profile.

Author notes
Funding Acknowledgements: None.



