-
PDF
- Split View
-
Views
-
Cite
Cite
Lale Tokgözoğlu, Angela Pirillo, Alberico Luigi Catapano, Glycerol and β-hydroxybutyrate: friends or foes?, European Heart Journal, Volume 44, Issue 39, 14 October 2023, Pages 4183–4185, https://doi.org/10.1093/eurheartj/ehad368
Close - Share Icon Share
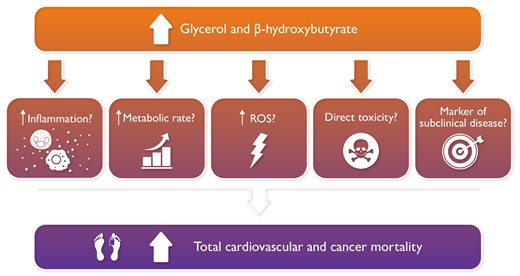
Could glycerol and β-hydroxybutyrate circulating levels be prognostic factors for mortality?
This editorial refers to ‘From plasma triglycerides to triglyceride metabolism: effects on mortality in the Copenhagen General Population Study’, by M.Ø. Johansen et al., https://doi.org/10.1093/eurheartj/ehad330.
Lipolysis is the process that generates glycerol and free fatty acids from the hydrolysis of triglycerides (TGs). Specific lipases are involved in this process, with lipoprotein lipase (LPL) playing a crucial role in determining the levels of circulating TGs at the endothelial interface, providing fuel for β-oxidation by mitochondria. Lipase activity is finely regulated, therefore its dysfunction has been linked to the development of several pathological conditions, including type 2 diabetes, obesity, cardiovascular disease, and cancer. In the blood, TGs are transported in lipoproteins and their plasma levels are determined by the balance between the production and clearance of lipoproteins. Epidemiological and genetic evidence supports a causal role for elevated plasma TGs and an increased risk of atherosclerotic cardiovascular diseases.1 Reducing TGs is thus regarded as an approach to decrease such a risk, albeit some discussion has emerged on whether apolipoprotein B (apoB) would be a better proxy for this effect.2 In addition, high levels of TGs are believed to play a role in carcinogenesis, with evidence for different types of cancer, probably through the activation of cellular proliferation.3
In their study published in this issue of the European Heart Journal, Johansen and colleagues report that a higher rate of TG metabolism was associated with an increased risk of death for all-cause, cardiovascular disease, cancer, and other causes in 30 000 individuals from the Copenhagen General Population Study.4 In this study, the rate of triglyceride metabolism was estimated by measuring circulating levels of glycerol and β-hydroxybutyrate.4 Plasma levels of glycerol are regarded as a measure of TG metabolism since it derives from the breakdown of the TG molecule. On the other hand, the ketone body β-hydroxybutyrate derives from the metabolism of fatty acids, which, in turn, derive from, but not exclusively, the metabolism of TGs.
The main finding of this study, as reported by the authors, is that increased TG catabolism appears to be a risk factor for mortality independent of circulating TG levels. Although the statistical analysis in this study provides robust results due to the large size of the tested sample, it is counter-intuitive that a higher TG metabolism might be associated with increased mortality. Speeding up the metabolism of TGs has been proposed as an effective strategy to reduce the risks associated with high TG levels such as cardiovascular risk or the risk of pancreatitis in severe hypertriglyceridaemia. Further, LPL deficiency has been associated with an increased risk of developing some types of cancers5 and also with learning and memory impairment, suggesting a protective role for functional LPL in Alzheimer’s disease neuropathogenesis.6
The authors have provided some possible explanations for the observed association between higher TG metabolism and increased mortality. Among them, they suggested that glycerol and β-hydroxybutyrate may have deleterious effects.4 This hypothesis, however, conflicts with a large number of observations indicating, particularly for β-hydroxybutyrate, several beneficial effects.
In a normal fed state, the circulating level of ketone bodies is ∼50 µM; upon fasting or other conditions, it can increase to 1–8 mM, and even higher (up to 20 mM) under pathological conditions such as diabetic ketoacidosis.7 Ketone bodies, among which β-hydroxybutyrate is the most abundant, represent an alternative fuel that can provide energy under specific conditions; β-hydroxybutyrate also acts as a signalling molecule, capable of relieving oxidative stress and inflammation and exerting beneficial effects on the causative factors of diseases such as diabetes, Alzheimer’s dementia, heart failure, and cancer.7–9 Several mechanisms have been explored to explain such beneficial effects, including the maintenance of mitochondrial homeostasis, the inhibition of the NLRP3 inflammasome, the binding to a specific receptor which results in reduced lipolysis in adipocytes, and the regulation of fatty acid availability and metabolism.7 Of note, SGLT2 inhibitors increase lipolysis and promote the production of ketone bodies in the liver, with an overall positive effect on diabetic hearts.10 Mildly elevated ketone levels can promote fuel efficiency and reduce the formation of reactive oxygen species (ROS), which might improve cardiac function and the prognosis of heart failure. Altogether these observations support the beneficial effects of β-hydroxybutyrate, with infusion and oral administration of this compound being shown to improve cardiac function.7 We must highlight, however, that some studies have reported detrimental effects with long-term exposure to β-hydroxybutyrate.7 A retrospective study reported an association between circulating levels of β-hydroxybutyrate and cardiovascular events and all-cause mortality in haemodialysis patients.11 Elevated plasma β-hydroxybutyrate levels predicted adverse outcomes and disease progression in patients with arrhythmogenic cardiomyopathy; these patients had levels significantly higher than healthy volunteers (144 µM vs. 24 µM), with plasma β-hydroxybutyrate increasing progressively with the severity of the disease.12 It is likely that, in these conditions, the mechanism(s) that trigger the increase in β-hydroxybutyrate (such as malnutrition in haemodialysis patients, or extrahepatic ketogenesis in early-stage arrhythmogenic cardiopathy), rather than a direct detrimental effect of β-hydroxybutyrate, can explain these associations. We must also underline that individuals in the highest quartile of β-hydroxybutyrate (as well as glycerol) in the study by Johansen and colleagues had higher mean age compared with those in the first quartile; age may be a relevant factor in determining the observed associations. Furthermore, ketone metabolism is affected by diet and exercise, as well as by other conditions, which also represent possible confounding factors. Of note, a recent study has shown that elevated levels of ketone bodies in apparently healthy people are associated with an increased risk of cardiovascular death and cardiovascular events, suggesting that this parameter could serve as a possible biomarker.13
The study by Johansen and colleagues also reported an association between high β-hydroxybutyrate and increased cancer mortality.4 Ketone body metabolism is dysregulated in many types of cancer; cancer cells undergo metabolic reprogramming to sustain rapid cell proliferation through increased glucose uptake and utilization. During glucose starvation, cells use ketone bodies as a metabolic fuel, but several observations have suggested that cancer cells may be inefficient in using ketone bodies, which are used as precursors for lipid synthesis rather than for energy supply.14 Furthermore, ketogenic enzymes are down-regulated in tumour cells, which is associated with a worse prognosis.14 However, other studies have reported the up-regulation of ketogenic enzymes and an association between ketone body utilization and tumour aggressiveness.14 On the other hand, beneficial effects of ketone bodies and the ketogenic diet in cancer therapy have been reported, with ketone bodies inducing growth inhibition and a cytotoxic effect in various cancer cell lines.14 These observations suggest that the role of dysregulated ketone body metabolism might be strictly related to the cell type, grade, and stage of the tumour, warranting further studies to establish the mechanisms linking ketone bodies in various cancer types. The association between elevated levels of β-hydroxybutyrate and increased cancer death reported in the study by Johansen and colleagues needs to be analysed in more detail to reveal the underlying mechanisms.
The hydrolysis of TGs releases free fatty acids and glycerol into the blood and thus the latter can be considered a marker of TG metabolism. In their study, Johansen and colleagues observed that individuals in the highest quartile of glycerol levels had an increased risk of death (all-cause, cardiovascular, cancer, and other mortalities). As already mentioned above, these subjects had a higher mean age. Previous studies have suggested a prognostic value for glycerol, with the CANVAS trial reporting that elevated fasting glycerol at baseline was associated with incident hospitalization for heart failure, cardiovascular mortality, and all-cause mortality in diabetic patients.15 The authors hypothesized that the high glycerol levels may be a biomarker of hepatic dysfunction and/or renal impairment. Of note, baseline levels of β-hydroxybutyrate were not associated with these outcomes in the CANVAS study.15
In summary, many alternative explanations exist for the correlations described by the authors, the increased TG turnover being one possibility (Graphical Abstract). However, until a direct physiological study demonstrates that individuals with elevated glycerol and β-hydroxybutyrate have increased catabolism of plasma lipoproteins/TGs, the jury is still out and the enigma of the effects of TG-related molecules on morbidity and mortality continues.
Declarations
Disclosure of Interest
L.T. has received honoraria for lectures or advisory board from Abbott, Amgen, Astra Zeneca, Bayer, Daiichi Sankyo, Janssen, MSD, Novartis, Novo Nordisk, Sanofi, Pfizer, and Recordati; A.P. has nothing to disclose; A.L.C. has received honoraria, lecture fees, or research grants from Aegerion, Amgen, Amryt, Astrazeneca, Bayer, Daiichi-Sankyo, Eli Lilly, Genzyme, Ionis Pharmaceutical, Kowa, Mediolanum, Medscape, Menarini, Merck, Mylan, Novartis, PeerVoice, Pfizer, Recordati, Regeneron, Sanofi, Sigma-Tau, and The Corpus.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.



