-
PDF
- Split View
-
Views
-
Cite
Cite
Davide Capodanno, Dominick J Angiolillo, Personalised antiplatelet therapies for coronary artery disease: what the future holds, European Heart Journal, Volume 44, Issue 32, 21 August 2023, Pages 3059–3072, https://doi.org/10.1093/eurheartj/ehad362
Close - Share Icon Share
Abstract
Coronary artery disease (CAD) is one of the leading causes of death globally, and antiplatelet therapy is crucial for both its prevention and treatment. Antiplatelet drugs such as aspirin and P2Y12 inhibitors are commonly used to reduce the risk of thrombotic events, including myocardial infarction, stroke, and stent thrombosis. However, the benefits associated with the use of antiplatelet drugs also come with a risk of bleeding complications. The ever-growing understanding of the poor prognostic implications associated with bleeding has set the foundations for defining strategies that can mitigate such safety concern without any trade-off in antithrombotic protection. To this extent, personalised antiplatelet therapy has emerged as a paradigm that optimizes the balance between safety and efficacy by customizing treatment to the individual patient's needs and risk profile. Accurate risk stratification for both bleeding and thrombosis can aid in selecting the optimal antiplatelet therapy and prevent serious and life-threatening outcomes. Risk stratification has traditionally included clinical and demographic characteristics and has expanded to incorporate angiographic features and laboratory findings. The availability of bedside platelet function testing as well as rapid genotyping assays has also allowed for a more individualized selection of antiplatelet therapy. This review provides a comprehensive overview of the current state of the art and future trends in personalised antiplatelet therapy for patients with CAD, with emphasis on those presenting with an acute coronary syndrome and undergoing percutaneous coronary revascularization. The aim is to provide clinicians with a comprehensive understanding of personalised antiplatelet therapy and facilitate informed clinical decision-making.

Proposed strategies for tailoring antithrombotic therapy according to individual ischaemic and bleeding risk. Patients at high risk of ischemic events and low risk of bleeding may benefit from an intensified antiplatelet therapy regimen (e.g., escalation), while those at high risk of bleeding and low risk of ischemic events require a less intensive and more cautious approach (e.g, de-escalation). In patients at low risk of ischemic events and low risk of bleeding the benefits of antiplatelet therapy may not outweigh the risks, while patients who have high risks of both ischemic and bleeding events require a delicate balance, as the approach to managing their conditions must weigh the potential benefits against the potential risks. Abbreviations: PFT, platelet function testing. Adapted from Cao et al.1
Introduction
Coronary artery disease (CAD) is a leading global cause of death and antiplatelet therapy plays a critical role in its prevention and treatment.2 Aspirin and P2Y12 receptor inhibitors are commonly used antiplatelet drugs that lower the risk of thrombotic events across the spectrum of CAD manifestations.3,4 However, the benefits associated with the use of antiplatelet drugs also come with a risk of bleeding complications.5 The ever-growing understanding of the poor prognostic implications associated with bleeding, including increased mortality, has set the foundations for defining strategies that can mitigate such safety concern without any trade-off in antithrombotic protection.6,7 To this extent, personalised antiplatelet treatment regimens represent a developing concept that seeks to achieve a net benefit between safety and efficacy by tailoring treatment to each patient's unique needs and risk profile.8,9 The identification of patients’ risk for both bleeding and thrombosis can aid in the selection of the optimal antiplatelet therapy and reduce the incidence of serious adverse events.
Multiple clinical, demographic, angiographic, and laboratory factors have been identified as predictors of bleeding and thrombosis.10–15 By considering these factors, clinicians are better equipped to risk stratify patients and in turn select the antiplatelet treatment regimen with the best risk-benefit profile for a given individual. Platelet function testing (PFT) and rapid genotyping are emerging as advancements that enable the assessment of a patient's individual response to antiplatelet therapy, allowing for an even more individualized selection of antiplatelet therapy.16 This review provides an overview of current state of the art and future trends in the role of risk assessment and personalised approach in antiplatelet therapies for patients with CAD, with emphasis on those presenting with an acute coronary syndrome (ACS) and undergoing percutaneous coronary revascularization. The review also highlights the growing field of personalised antiplatelet therapy, including PFT and genotyping, and discusses their future directions and potential implications.
Antiplatelet therapies in cardiovascular disease
Mechanisms of action of oral and intravenous antiplatelet drugs currently approved for clinical use are visualized in Figure 1.17
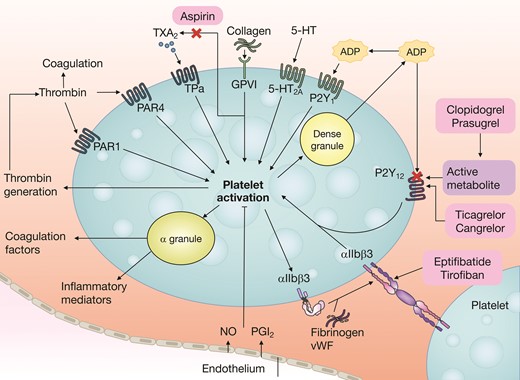
Platelet activation mechanisms. Platelet activation is initiated by soluble agonists, such as thrombin, thromboxane A2 (TXA2), 5-hydroxytryptamine (5-HT) and ADP (via the P2Y1 receptor), and by adhesive ligands, such as collagen and von Willebrand factor (vWF). Consequently, dense granule secretion of platelet agonists and secretion of TXA2, as a result of phospholipase A2 activation, lead to the amplification of platelet activation and the associated responses. The P2Y12 receptor has a major role in the amplification of platelet activation, which is also supported by outside–in signalling via integrin αIIbβ3 (the glycoprotein IIb/IIIa receptor). Aspirin inhibits platelet function by acetylation of the platelet cyclooxygenase, which prevents the access of arachidonic acid to the catalytic site of the enzyme and results in irreversible inhibition of platelet dependent TXA2 formation. Therefore, the combined use of aspirin and a P2Y12 receptor inhibitor (such as clopidogrel, prasugrel, or ticagrelor) has additive effects on the inhibition of platelet activation and the associated platelet responses. Conversely, discontinuation of aspirin results in some degree of increased platelet reactivity driven by pathways mediated by arachidonic acid and collagen, whereas other pathways remain inhibited by P2Y12 inhibitor monotherapy. 5-HT2A, 5-hydroxytryptamine receptor 2A; GPVI, platelet glycoprotein VI; NO, nitric oxide; PAR, proteinase-activated receptor; PGI2, prostaglandin I2; TPα, thromboxane A2 receptor isoform α. Reproduced with permission from Capodanno et al.17
In accordance with type I classes of recommendations from practice guidelines,3,4 aspirin is generally indicated for the secondary prevention of CAD, including patients with previous myocardial infarction (MI) or revascularization, and the P2Y12 inhibitor clopidogrel is an alternative to aspirin for patients with allergy or intolerance. Additionally, clopidogrel is prescribed after percutaneous coronary intervention (PCI) in patients who present with a chronic coronary syndrome (CCS). Prasugrel is recommended in patients with an ACS undergoing PCI and ticagrelor in patients with ACS with or without revascularization. A description on the use of oral anticoagulants in patients with CAD and antiplatelet therapy in patients on oral anticoagulants goes beyond the scope of this manuscript and is described elsewhere.18–20
Risk assessment
The 2021 guidelines from the European Society of Cardiology (ESC) for cardiovascular disease prevention in clinical practice emphasize the importance of timely recognition of risk factors, predictors, and modifiers that impact the likelihood of CAD complications.3 This paradigm can be easily extended to the risks confronted by patients on antiplatelet therapy. In fact, the key to thrombosis and bleeding prevention is to identify those patients who will receive the greatest benefit from antiplatelet drugs at the least possible safety price.
In general, the higher the absolute risk of CAD, the higher the expected absolute benefit of antiplatelet therapy in reducing thrombotic complications (e.g. MI, stroke, stent thrombosis). However, antiplatelet therapy carries an unavoidable risk of bleeding, which can be mitigated by weighing the benefits and risks for the individual patient and adjusting the treatment plan accordingly. Therefore, determining the fine balance between thrombotic and bleeding risks is crucial for the effective management of antiplatelet therapy.
Predictors of thrombotic and bleeding events
Risk stratification tools for the prediction of ischemia, bleeding, or their trade-off in CAD patients on dual antiplatelet therapy (DAPT, i.e. the combination of aspirin and a P2Y12 inhibitor) are summarized in Table 1. 10–15 These models were generally derived from large cohorts of patients undergoing PCI and therefore their generalizability to other DAPT settings (i.e. CAD without PCI) is uncertain.
Risk stratification tools for ischemia, bleeding, or their trade-off in patients on dual antiplatelet therapy
| Score/Model . | No of variables . | Development cohort (patients, design) . | Setting . | Predicted outcome . | Validation cohort(s) (patients, c-index) . |
|---|---|---|---|---|---|
| Ischemia | |||||
| PARIS thrombosis10 | Six clinical | 4190 patients, multicentre registry | PCI patients on DAPT | Ischemia at 24 months after PCI | 8665 patients, 0.65 |
| Bleeding | |||||
| PARIS bleeding10 | Six clinical | 4190 patients, multicentre registry | PCI patients on DAPT | Bleeding at 24 months after PCI | 8665 patients, 0.64 |
| PRECISE-DAPT11 | Five clinical | 14 963 patients, pooled analysis of randomised clinical trials | PCI patients on DAPT | Bleeding at 12 months after PCI | 8595 patients, 0.70; 6172 patients, 0.66 |
| BleeMACS12 | Seven clinical | 15 401 patients, multicentre registry | PCI patients on DAPT | Bleeding at 12 months after PCI | 96 239 patients, 0.65 |
| ARC-HBR13 | Thirteen clinical | - | PCI patients on DAPT | Bleeding at 12 months after PCI | - |
| Trade-off | |||||
| DAPT score14 | Five clinical, three procedural | 11 648 patients, multicentre randomised clinical trial | PCI patients on DAPT who were event-free for 12 months | Ischemia and bleeding between 12 and 30 months after PCI | 8136 patients, 0.64 for both ischaemia and bleeding |
| ARC-HBR trade-off15 | Ten clinical, two procedural | 6641 patients, pooled analysis of randomised clinical trials and a multicentre registry | PCI patients on DAPT | Ischemia and bleeding at 12 months after PCI | 1458 patients, 0.74 for both ischaemia and bleeding |
| Score/Model . | No of variables . | Development cohort (patients, design) . | Setting . | Predicted outcome . | Validation cohort(s) (patients, c-index) . |
|---|---|---|---|---|---|
| Ischemia | |||||
| PARIS thrombosis10 | Six clinical | 4190 patients, multicentre registry | PCI patients on DAPT | Ischemia at 24 months after PCI | 8665 patients, 0.65 |
| Bleeding | |||||
| PARIS bleeding10 | Six clinical | 4190 patients, multicentre registry | PCI patients on DAPT | Bleeding at 24 months after PCI | 8665 patients, 0.64 |
| PRECISE-DAPT11 | Five clinical | 14 963 patients, pooled analysis of randomised clinical trials | PCI patients on DAPT | Bleeding at 12 months after PCI | 8595 patients, 0.70; 6172 patients, 0.66 |
| BleeMACS12 | Seven clinical | 15 401 patients, multicentre registry | PCI patients on DAPT | Bleeding at 12 months after PCI | 96 239 patients, 0.65 |
| ARC-HBR13 | Thirteen clinical | - | PCI patients on DAPT | Bleeding at 12 months after PCI | - |
| Trade-off | |||||
| DAPT score14 | Five clinical, three procedural | 11 648 patients, multicentre randomised clinical trial | PCI patients on DAPT who were event-free for 12 months | Ischemia and bleeding between 12 and 30 months after PCI | 8136 patients, 0.64 for both ischaemia and bleeding |
| ARC-HBR trade-off15 | Ten clinical, two procedural | 6641 patients, pooled analysis of randomised clinical trials and a multicentre registry | PCI patients on DAPT | Ischemia and bleeding at 12 months after PCI | 1458 patients, 0.74 for both ischaemia and bleeding |
ARC-HBR, Academic Research Consortium for High Bleeding Risk; BleeMACS, Bleeding Complications in a Multicenter Registry of Patients Discharged With Diagnosis of Acute Coronary Syndrome; DAPT, Dual Antiplatelet Therapy Trial; PARIS, patterns of non-adherence to anti-platelet regimens in stented patients; PCI, percutaneous coronary intervention; PRECISE-DAPT, Predicting Bleeding Complications In Patients Undergoing Stent Implantation and Subsequent Dual Anti Platelet Therapy.
Risk stratification tools for ischemia, bleeding, or their trade-off in patients on dual antiplatelet therapy
| Score/Model . | No of variables . | Development cohort (patients, design) . | Setting . | Predicted outcome . | Validation cohort(s) (patients, c-index) . |
|---|---|---|---|---|---|
| Ischemia | |||||
| PARIS thrombosis10 | Six clinical | 4190 patients, multicentre registry | PCI patients on DAPT | Ischemia at 24 months after PCI | 8665 patients, 0.65 |
| Bleeding | |||||
| PARIS bleeding10 | Six clinical | 4190 patients, multicentre registry | PCI patients on DAPT | Bleeding at 24 months after PCI | 8665 patients, 0.64 |
| PRECISE-DAPT11 | Five clinical | 14 963 patients, pooled analysis of randomised clinical trials | PCI patients on DAPT | Bleeding at 12 months after PCI | 8595 patients, 0.70; 6172 patients, 0.66 |
| BleeMACS12 | Seven clinical | 15 401 patients, multicentre registry | PCI patients on DAPT | Bleeding at 12 months after PCI | 96 239 patients, 0.65 |
| ARC-HBR13 | Thirteen clinical | - | PCI patients on DAPT | Bleeding at 12 months after PCI | - |
| Trade-off | |||||
| DAPT score14 | Five clinical, three procedural | 11 648 patients, multicentre randomised clinical trial | PCI patients on DAPT who were event-free for 12 months | Ischemia and bleeding between 12 and 30 months after PCI | 8136 patients, 0.64 for both ischaemia and bleeding |
| ARC-HBR trade-off15 | Ten clinical, two procedural | 6641 patients, pooled analysis of randomised clinical trials and a multicentre registry | PCI patients on DAPT | Ischemia and bleeding at 12 months after PCI | 1458 patients, 0.74 for both ischaemia and bleeding |
| Score/Model . | No of variables . | Development cohort (patients, design) . | Setting . | Predicted outcome . | Validation cohort(s) (patients, c-index) . |
|---|---|---|---|---|---|
| Ischemia | |||||
| PARIS thrombosis10 | Six clinical | 4190 patients, multicentre registry | PCI patients on DAPT | Ischemia at 24 months after PCI | 8665 patients, 0.65 |
| Bleeding | |||||
| PARIS bleeding10 | Six clinical | 4190 patients, multicentre registry | PCI patients on DAPT | Bleeding at 24 months after PCI | 8665 patients, 0.64 |
| PRECISE-DAPT11 | Five clinical | 14 963 patients, pooled analysis of randomised clinical trials | PCI patients on DAPT | Bleeding at 12 months after PCI | 8595 patients, 0.70; 6172 patients, 0.66 |
| BleeMACS12 | Seven clinical | 15 401 patients, multicentre registry | PCI patients on DAPT | Bleeding at 12 months after PCI | 96 239 patients, 0.65 |
| ARC-HBR13 | Thirteen clinical | - | PCI patients on DAPT | Bleeding at 12 months after PCI | - |
| Trade-off | |||||
| DAPT score14 | Five clinical, three procedural | 11 648 patients, multicentre randomised clinical trial | PCI patients on DAPT who were event-free for 12 months | Ischemia and bleeding between 12 and 30 months after PCI | 8136 patients, 0.64 for both ischaemia and bleeding |
| ARC-HBR trade-off15 | Ten clinical, two procedural | 6641 patients, pooled analysis of randomised clinical trials and a multicentre registry | PCI patients on DAPT | Ischemia and bleeding at 12 months after PCI | 1458 patients, 0.74 for both ischaemia and bleeding |
ARC-HBR, Academic Research Consortium for High Bleeding Risk; BleeMACS, Bleeding Complications in a Multicenter Registry of Patients Discharged With Diagnosis of Acute Coronary Syndrome; DAPT, Dual Antiplatelet Therapy Trial; PARIS, patterns of non-adherence to anti-platelet regimens in stented patients; PCI, percutaneous coronary intervention; PRECISE-DAPT, Predicting Bleeding Complications In Patients Undergoing Stent Implantation and Subsequent Dual Anti Platelet Therapy.
The PARIS risk score encompasses six clinical variables for the prediction of thrombosis and six variables for the prediction of bleeding.10 Other scores for bleeding include PRECISE-DAPT,11 which uses five clinical predictors, and BleeMACS, which uses seven clinical predictors.12 While these models follow a quantitative approach, a semiquantitative approach from the Academic Research Consortium for High Bleeding Risk (ARC-HBR) has recently been introduced,13 with patients at high bleeding risk defined as those fulfilling one major criterion or two minor criteria from a set of variables defined by expert consensus. Two models (DAPT score and ARC-HBR trade-off model) are also available that integrate ischemic and bleeding risk factors to provide a prediction rule for outcomes of ‘net benefit’.14,15
Overall, the discriminatory performance of the available risk models for ischemia and/or bleeding is in the range of c-statistics between 0.65 and 0.75 in validation studies10–15,21, indicating their suboptimal capacity to distinguish the patients who will experience an event from and those who will not. Importantly, high bleeding risk patients have often been excluded from study populations used to derive bleeding risk scores, and these scores have been evaluated in the context of standard DAPT, which makes their generalisability uncertain when other scenarios are considered (e.g. de-escalation). Notably, the ESC guidelines assign a low class of recommendation to the use of risk scores to guide antiplatelet therapy (e.g. class IIb in the European guidelines for ACS).22 Therefore, clinical considerations and sound clinical judgment are crucial on top of utilizing these predictive models in guiding patient management and treatment decisions.21 Yet, identifying patients who are most likely to benefit from antiplatelet therapy (e.g. those at very high risk of CAD events) and have a low risk of bleeding is challenging, especially because patients at increased ischemic and bleeding risks typically share common CAD risk factors.15,23
Additionally, patients with established atherosclerotic cardiovascular disease are generally considered at very high risk of events by current ESC guidelines for secondary prevention.3 This broad group include those with documented CAD, including previous MI, ACS, and coronary revascularization. Patients with unequivocally documented cardiovascular disease on imaging are also considered at very high risk, including those with atherosclerotic plaques on coronary angiography or computed tomography angiography.
A scrutiny of most common predictors derived by the available risk stratification tools (Table 2) allows to isolate some crucial risk determinants of ischemic complications in spite of antiplatelet therapy (e.g. diabetes mellitus, ACS or MI at presentation, prior PCI, MI or cerebrovascular accident, smoking, renal insufficiency, peripheral artery disease) and a number of key predictors of bleeding (e.g. age, renal insufficiency, cirrhosis, anaemia or low haemoglobin levels, oral anticoagulation, prior bleeding and/or transfusion, cancer) that are visually schematized in Figure 2.
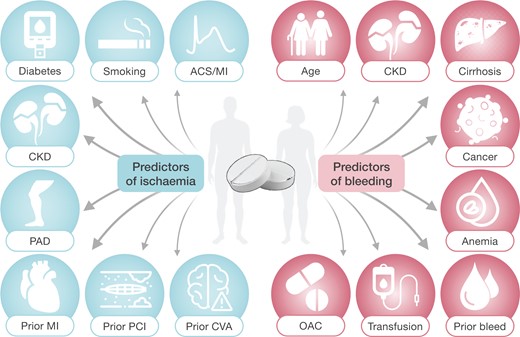
Determinants of ischaemic and bleeding risk in patients on dual antiplatelet therapy. Abbreviations: ACS, acute coronary syndrome; CKD, chronic kidney disease; CVA, cerebrovascular accidents; MI, myocardial infarction; OAC, oral anticoagulation; PAD, peripheral artery disease; PCI, percutaneous coronary intervention.
Predictors of ischemia and/or bleeding in patients on dual antiplatelet therapy
| . | PARIS10 . | PRECISE DAPT11 . | BleeMACS12 . | ARC-HBR13 . | DAPT score14 . | ARC-HBR Trade-off15 . |
|---|---|---|---|---|---|---|
| Predictors of ischemia | ||||||
| Diabetes | ◉ | NA | NA | NA | ◉ | ◉ |
| Smoking | ◉ | NA | NA | NA | ◉ | ◉ |
| ACS or MI at presentation | ◉ | NA | NA | NA | ◉ | ◉ |
| Prior PCI or MI | ◉ | NA | NA | NA | ◉ | ◉ |
| Renal insufficiency | ◉ | NA | NA | NA | ◉ | ◉ |
| Prior CABG | ◉ | NA | NA | NA | – | – |
| Small stents | – | NA | NA | NA | ◉ | – |
| CHF or low LVEF | – | NA | NA | NA | ◉ | – |
| Paclitaxel-eluting stent | – | NA | NA | NA | ◉ | – |
| Vein graft stent | – | NA | NA | NA | ◉ | – |
| Vascular disease | – | NA | NA | NA | ◉ | – |
| Hypertension | – | NA | NA | NA | ◉ | – |
| Anaemia or haemoglobin | – | NA | NA | NA | – | ◉ |
| Complex PCI | – | NA | NA | NA | – | ◉ |
| Bare metal stent | – | NA | NA | NA | – | ◉ |
| Predictors of bleeding | ||||||
| Age | ◉ | ◉ | ◉ | ◉ | ◉ | ◉ |
| Renal insufficiency | ◉ | ◉ | ◉ | ◉ | ◉ | ◉ |
| Anaemia or low haemoglobin | ◉ | ◉ | ◉ | ◉ | – | ◉ |
| Oral anticoagulation | ◉ | – | – | ◉ | – | ◉ |
| Prior bleeding and/or transfusion | – | ◉ | ◉ | ◉ | – | – |
| Cancer | – | – | ◉ | ◉ | – | ◉ |
| Smoking | ◉ | – | – | – | – | ◉ |
| Liver cirrhosis | – | – | – | ◉ | – | ◉ |
| Planned major noncardiac surgery | – | – | – | ◉ | – | ◉ |
| Hypertension | – | – | ◉ | – | ◉ | – |
| Vascular disease | – | – | ◉ | – | ◉ | – |
| Low or high body mass index | ◉ | – | – | – | – | – |
| Leucocytosis | – | ◉ | – | – | – | – |
| Thrombocytopenia | – | – | – | ◉ | – | – |
| Chronic bleeding diathesis | – | – | – | ◉ | – | – |
| Long-term use of NSAIDs or steroids | – | – | – | ◉ | – | – |
| Previous ischemic stroke or ICH | – | – | – | ◉ | – | – |
| Recent major surgery or trauma | – | – | – | ◉ | – | – |
| COPD | – | – | – | – | – | ◉ |
| Complex PCI | – | – | – | – | – | ◉ |
| . | PARIS10 . | PRECISE DAPT11 . | BleeMACS12 . | ARC-HBR13 . | DAPT score14 . | ARC-HBR Trade-off15 . |
|---|---|---|---|---|---|---|
| Predictors of ischemia | ||||||
| Diabetes | ◉ | NA | NA | NA | ◉ | ◉ |
| Smoking | ◉ | NA | NA | NA | ◉ | ◉ |
| ACS or MI at presentation | ◉ | NA | NA | NA | ◉ | ◉ |
| Prior PCI or MI | ◉ | NA | NA | NA | ◉ | ◉ |
| Renal insufficiency | ◉ | NA | NA | NA | ◉ | ◉ |
| Prior CABG | ◉ | NA | NA | NA | – | – |
| Small stents | – | NA | NA | NA | ◉ | – |
| CHF or low LVEF | – | NA | NA | NA | ◉ | – |
| Paclitaxel-eluting stent | – | NA | NA | NA | ◉ | – |
| Vein graft stent | – | NA | NA | NA | ◉ | – |
| Vascular disease | – | NA | NA | NA | ◉ | – |
| Hypertension | – | NA | NA | NA | ◉ | – |
| Anaemia or haemoglobin | – | NA | NA | NA | – | ◉ |
| Complex PCI | – | NA | NA | NA | – | ◉ |
| Bare metal stent | – | NA | NA | NA | – | ◉ |
| Predictors of bleeding | ||||||
| Age | ◉ | ◉ | ◉ | ◉ | ◉ | ◉ |
| Renal insufficiency | ◉ | ◉ | ◉ | ◉ | ◉ | ◉ |
| Anaemia or low haemoglobin | ◉ | ◉ | ◉ | ◉ | – | ◉ |
| Oral anticoagulation | ◉ | – | – | ◉ | – | ◉ |
| Prior bleeding and/or transfusion | – | ◉ | ◉ | ◉ | – | – |
| Cancer | – | – | ◉ | ◉ | – | ◉ |
| Smoking | ◉ | – | – | – | – | ◉ |
| Liver cirrhosis | – | – | – | ◉ | – | ◉ |
| Planned major noncardiac surgery | – | – | – | ◉ | – | ◉ |
| Hypertension | – | – | ◉ | – | ◉ | – |
| Vascular disease | – | – | ◉ | – | ◉ | – |
| Low or high body mass index | ◉ | – | – | – | – | – |
| Leucocytosis | – | ◉ | – | – | – | – |
| Thrombocytopenia | – | – | – | ◉ | – | – |
| Chronic bleeding diathesis | – | – | – | ◉ | – | – |
| Long-term use of NSAIDs or steroids | – | – | – | ◉ | – | – |
| Previous ischemic stroke or ICH | – | – | – | ◉ | – | – |
| Recent major surgery or trauma | – | – | – | ◉ | – | – |
| COPD | – | – | – | – | – | ◉ |
| Complex PCI | – | – | – | – | – | ◉ |
Abbreviations: ACS, acute coronary syndromes; CABG, coronary artery bypass surgery; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; ICH, intracranial haemorrhage; LVEF, low ventricular ejection fraction, MI, myocardial infarction; NA, not applicable; NSAIDs, nonsteroidal anti-inflammatory drugs; PCI, percutaneous coronary intervention. Other abbreviations as in Table 1.
Predictors of ischemia and/or bleeding in patients on dual antiplatelet therapy
| . | PARIS10 . | PRECISE DAPT11 . | BleeMACS12 . | ARC-HBR13 . | DAPT score14 . | ARC-HBR Trade-off15 . |
|---|---|---|---|---|---|---|
| Predictors of ischemia | ||||||
| Diabetes | ◉ | NA | NA | NA | ◉ | ◉ |
| Smoking | ◉ | NA | NA | NA | ◉ | ◉ |
| ACS or MI at presentation | ◉ | NA | NA | NA | ◉ | ◉ |
| Prior PCI or MI | ◉ | NA | NA | NA | ◉ | ◉ |
| Renal insufficiency | ◉ | NA | NA | NA | ◉ | ◉ |
| Prior CABG | ◉ | NA | NA | NA | – | – |
| Small stents | – | NA | NA | NA | ◉ | – |
| CHF or low LVEF | – | NA | NA | NA | ◉ | – |
| Paclitaxel-eluting stent | – | NA | NA | NA | ◉ | – |
| Vein graft stent | – | NA | NA | NA | ◉ | – |
| Vascular disease | – | NA | NA | NA | ◉ | – |
| Hypertension | – | NA | NA | NA | ◉ | – |
| Anaemia or haemoglobin | – | NA | NA | NA | – | ◉ |
| Complex PCI | – | NA | NA | NA | – | ◉ |
| Bare metal stent | – | NA | NA | NA | – | ◉ |
| Predictors of bleeding | ||||||
| Age | ◉ | ◉ | ◉ | ◉ | ◉ | ◉ |
| Renal insufficiency | ◉ | ◉ | ◉ | ◉ | ◉ | ◉ |
| Anaemia or low haemoglobin | ◉ | ◉ | ◉ | ◉ | – | ◉ |
| Oral anticoagulation | ◉ | – | – | ◉ | – | ◉ |
| Prior bleeding and/or transfusion | – | ◉ | ◉ | ◉ | – | – |
| Cancer | – | – | ◉ | ◉ | – | ◉ |
| Smoking | ◉ | – | – | – | – | ◉ |
| Liver cirrhosis | – | – | – | ◉ | – | ◉ |
| Planned major noncardiac surgery | – | – | – | ◉ | – | ◉ |
| Hypertension | – | – | ◉ | – | ◉ | – |
| Vascular disease | – | – | ◉ | – | ◉ | – |
| Low or high body mass index | ◉ | – | – | – | – | – |
| Leucocytosis | – | ◉ | – | – | – | – |
| Thrombocytopenia | – | – | – | ◉ | – | – |
| Chronic bleeding diathesis | – | – | – | ◉ | – | – |
| Long-term use of NSAIDs or steroids | – | – | – | ◉ | – | – |
| Previous ischemic stroke or ICH | – | – | – | ◉ | – | – |
| Recent major surgery or trauma | – | – | – | ◉ | – | – |
| COPD | – | – | – | – | – | ◉ |
| Complex PCI | – | – | – | – | – | ◉ |
| . | PARIS10 . | PRECISE DAPT11 . | BleeMACS12 . | ARC-HBR13 . | DAPT score14 . | ARC-HBR Trade-off15 . |
|---|---|---|---|---|---|---|
| Predictors of ischemia | ||||||
| Diabetes | ◉ | NA | NA | NA | ◉ | ◉ |
| Smoking | ◉ | NA | NA | NA | ◉ | ◉ |
| ACS or MI at presentation | ◉ | NA | NA | NA | ◉ | ◉ |
| Prior PCI or MI | ◉ | NA | NA | NA | ◉ | ◉ |
| Renal insufficiency | ◉ | NA | NA | NA | ◉ | ◉ |
| Prior CABG | ◉ | NA | NA | NA | – | – |
| Small stents | – | NA | NA | NA | ◉ | – |
| CHF or low LVEF | – | NA | NA | NA | ◉ | – |
| Paclitaxel-eluting stent | – | NA | NA | NA | ◉ | – |
| Vein graft stent | – | NA | NA | NA | ◉ | – |
| Vascular disease | – | NA | NA | NA | ◉ | – |
| Hypertension | – | NA | NA | NA | ◉ | – |
| Anaemia or haemoglobin | – | NA | NA | NA | – | ◉ |
| Complex PCI | – | NA | NA | NA | – | ◉ |
| Bare metal stent | – | NA | NA | NA | – | ◉ |
| Predictors of bleeding | ||||||
| Age | ◉ | ◉ | ◉ | ◉ | ◉ | ◉ |
| Renal insufficiency | ◉ | ◉ | ◉ | ◉ | ◉ | ◉ |
| Anaemia or low haemoglobin | ◉ | ◉ | ◉ | ◉ | – | ◉ |
| Oral anticoagulation | ◉ | – | – | ◉ | – | ◉ |
| Prior bleeding and/or transfusion | – | ◉ | ◉ | ◉ | – | – |
| Cancer | – | – | ◉ | ◉ | – | ◉ |
| Smoking | ◉ | – | – | – | – | ◉ |
| Liver cirrhosis | – | – | – | ◉ | – | ◉ |
| Planned major noncardiac surgery | – | – | – | ◉ | – | ◉ |
| Hypertension | – | – | ◉ | – | ◉ | – |
| Vascular disease | – | – | ◉ | – | ◉ | – |
| Low or high body mass index | ◉ | – | – | – | – | – |
| Leucocytosis | – | ◉ | – | – | – | – |
| Thrombocytopenia | – | – | – | ◉ | – | – |
| Chronic bleeding diathesis | – | – | – | ◉ | – | – |
| Long-term use of NSAIDs or steroids | – | – | – | ◉ | – | – |
| Previous ischemic stroke or ICH | – | – | – | ◉ | – | – |
| Recent major surgery or trauma | – | – | – | ◉ | – | – |
| COPD | – | – | – | – | – | ◉ |
| Complex PCI | – | – | – | – | – | ◉ |
Abbreviations: ACS, acute coronary syndromes; CABG, coronary artery bypass surgery; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; ICH, intracranial haemorrhage; LVEF, low ventricular ejection fraction, MI, myocardial infarction; NA, not applicable; NSAIDs, nonsteroidal anti-inflammatory drugs; PCI, percutaneous coronary intervention. Other abbreviations as in Table 1.
Personalised approach
Tailoring the right antiplatelet therapy to individual ischemic and bleeding characteristics is crucial in optimizing the benefits and minimizing the risks of treatment for patients with CAD (Graphical abstract).1 Patients at high risk of ischemic events and low risk of bleeding may benefit from a more intensive antiplatelet therapy regimen, while those at high risk of bleeding and low risk of ischemic events require a less intensive and more cautious approach. In patients at low risk of ischemic events and low risk of bleeding, the benefits of antiplatelet therapy may not outweigh the risks, while patients who have high risks of both ischemic and bleeding events require a delicate balance, as the approach to managing their conditions must weigh the potential benefits against the potential risks.
There are two potential strategies to personalising the antiplatelet treatment regimen based on the individual risk of ischemic and bleeding complications.8 The first strategy is an approach based on clinical judgment, where the practitioner uses commonly available parameters, including clinical, demographic, laboratory, and procedural, along with their experience to make decisions. The second strategy is an approach based on guidance from assays that may inform on how a patient is responding (i.e. PFT) or may respond (i.e. genetic testing) to an antiplatelet agent. This may include results from PFT that measure the individual’s response to an antiplatelet drug which correlates with an adverse outcome (e.g. hyper- and hypo-responders with bleeding and thrombotic events, respectively). Alternatively, genetic testing provides information about how an individual may respond to a given antiplatelet agent (e.g. genetic polymorphisms coding for an enzyme involved in drug metabolism may lead to different degrees of enzyme activity). Both strategies aim to optimize the benefits of antiplatelet therapy in reducing the risk of ischemic complications and mitigate the risk of bleeding complications.
Clinically guided approach
The standard approach to antiplatelet therapy in patients with CAD is to prescribe 6 months of DAPT after PCI for CCS and 12 months after an ACS with or without PCI.22,24–27 In particular, in addition to aspirin, clopidogrel is the P2Y12 inhibitor of choice in patients with CCS, while prasugrel (in patients undergoing PCI) and ticagrelor (regardless of revascularization) are preferred over clopidogrel in patients with ACS, with ESC guidelines recommending prasugrel over ticagrelor in patients undergoing PCI.22,28 Indeed, the duration of DAPT can be prolonged or shortened according the ischemic and bleeding risk profile of the patient. In patients who are at increased risk for both ischemic and bleeding complications, bleeding, more than ischemic risk or PCI complexity, should inform decision-making on the duration of DAPT.29 After completion of DAPT, patients should resume single antiplatelet therapy (SAPT). Aspirin has been the standard of care for SAPT in most patients with CAD. However, there is emerging evidence in the post-PCI setting supporting the use of a P2Y12 inhibitor as chronic monotherapy instead.30,31
In addition to variations in the duration of DAPT, the availability of different oral antiplatelet agents as well as a better understanding of the timing at which patients may be at an enhanced risk of thrombotic or bleeding complications has led strategies of modulation of the intensity of antiplatelet therapy as an approach to optimize the safety–efficacy balance in an individual patient. In particular, modulation of the antiplatelet treatment regimen may occur in two ways: by reducing (i.e. de-escalation) or increasing (i.e. escalation) the intensity of platelet inhibition (Figure 3).32,33Table 3 summarizes the results of contemporary randomized clinical trials of antiplatelet therapy modulation. The results of these studies can guide a patient-centric approach where physicians use their clinical judgment and expertise to determine which candidates are best suited for a specific investigational treatment plan.
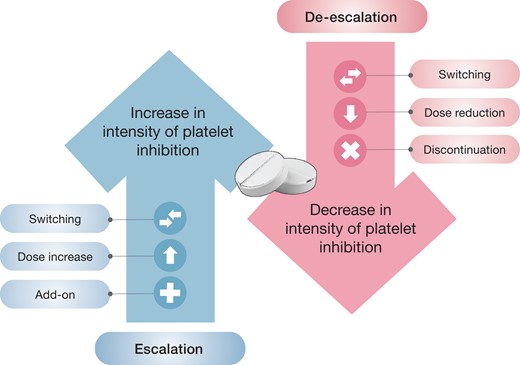
Strategies of antiplatelet therapy modulation. Antiplatelet therapy can be modulated in two ways: by reducing (i.e. de-escalation) or increasing (i.e. escalation) the intensity of platelet inhibition.
Contemporary randomized controlled trials of unselective modulation of antiplatelet therapy
| Trial . | Modulation . | Strategy . | n . | Population . | Follow-up . | Primary endpoint . | Findings . |
|---|---|---|---|---|---|---|---|
| TOPIC34 | De-escalation | Switch from prasugrel or ticagrelor to clopidogrel | 646 | ACS and PCI | 12 months | NACE | HR, 0.48; 95% CI, 0.34–0.68; P < 0.01 |
| TALOS-AMI35 | De-escalation | Switch from ticagrelor to clopidogrel | 2697 | Prior ACS and PCI | 11 months | NACE | HR, 0.55; 95% CI, 0.40–0.76; P = 0.0001 |
| HOST-REDUCE36 | De-escalation | Dose reduction from prasugrel 10 to 5 mg once daily | 2338 | ACS and PCI | 12 months | NACE | HR, 0.70; 95% CI, 0.52–0.92; P = 0.012 |
| ONE-MONTH DAPT37 | De-escalation | Discontinuation of the P2Y12 inhibitor (1 vs. 6–12 months of DAPT) | 3020 | CCS and PCI | 12 months | NACE | ARD, -0.7%; upper limit of 1-sided 97.5% CI, 1.33%; P < 0.001 for noninferiority |
| SMART-DATE38 | De-escalation | Discontinuation of the P2Y12 inhibitor (6 vs. 12 months of DAPT) | 2712 | ACS and PCI | 18 months | MACE | ARD, 0.5%; upper limit of 1-sided 95% CI, 1.8%; P = 0.03 for noninferiority |
| REDUCE ACS39 | De-escalation | Discontinuation of the P2Y12 inhibitor (3 vs. 12 months of DAPT) | 1496 | ACS and PCI | 12 months | NACE | ARD, -0.0022%; upper limit of 1-sided 95% CI, 0.027%; P < 0.001 for noninferiority |
| DAPT STEMI40 | De-escalation | Discontinuation of the P2Y12 inhibitor (6 vs. 12 months of DAPT) | 1100 | Prior ACS and PCI | 18 months | NACE | HR, 0.73; 95% CI 0.41–1.27; P = 0.004 for noninferiority |
| GLOBAL LEADERS41 | De-escalation | Discontinuation of aspirin (1 vs. 12 months of DAPT) | 15 968 | PCI | 24 months | MACE | Rate ratio, 0.87; 95% CI, 0.75–1.01; P = 0.073) |
| TWILIGHT42 | De-escalation | Discontinuation of aspirin (3 vs. 12 months of DAPT) | 7119 | PCI | 12 months | Bleeding | HR, 0.56; 95% CI, 0.45–0.68; P < 0.001 |
| STOPDAPT-243 | De-escalation | Discontinuation of aspirin (1 vs. 12 months of DAPT) | 3045 | PCI | 12 months | NACE | HR, 0.64; 95% CI, 0.42–0.98; P = 0.04 |
| SMART-CHOICE44 | De-escalation | Discontinuation of aspirin (3 vs. 12 months of DAPT) | 2993 | PCI | 12 months | MACE | ARD, 0.4%, upper limit of 1-sided 95% CI, 1.3%; P = 0.007 for noninferiority |
| TICO45 | De-escalation | Discontinuation of aspirin (3 vs. 12 months of DAPT) | 3056 | ACS | 12 months | NACE | HR, 0.66; 95% CI, 0.48–0.92; P = 0.01 |
| STOPDAPT-2 ACS46 | De-escalation | Discontinuation of aspirin (1–2 vs. 12 months of DAPT) | 4169 | ACS | 12 months | NACE | HR, 1.14; 95% CI, 0.80–1.62; P = 0.06 for noninferiority |
| MASTER DAPT47 | De-escalation | Discontinuation of aspirin or the P2Y12 inhibitor (1 vs. ≥3 months of DAPT) | 4434 | PCI | 11 months | NACE | ARD, -0.23%, upper limit of 95% CI, 1.33%; P < 0.001 for noninferiority |
| HOST-IDEA48 | De-escalation | Discontinuation or the P2Y12 inhibitor (3–6 vs. 12 months of DAPT) | 2013 | PCI | 12 months | NACE | ARD, -0.4%, upper limit of 1-sided 95%, 1.1%; P < 0.001 for noninferiority |
| DAPT49 | Escalation | Add-on P2Y12 inhibitor (30 vs. 12gr months of DAPT) | 9961 | Prior PCI | 18 months | MACE | HR, 0.71; 95% CI, 0.59–0.85; P < 0.001 |
| PEGASUS-TIMI 5450 | Escalation | Add-on ticagrelor (long-term DAPT vs. aspirin) | 21 162 | Prior MI | ∼33 months | MACE | HR (ticagrelor 90 mg), 0.85; 95% CI, 0.75–0.96; P = 0.008; HR (ticagrelor 60 mg), 0.84; 95% CI, 0.74–0.95; P = 0.004 |
| THEMIS51 | Escalation | Add-on ticagrelor (long-term DAPT vs. aspirin) | 19 220 | CCS and diabetes | ∼40 months | MACE | HR, 0.90; 95% CI, 0.81–0.99; P = 0.04 |
| Trial . | Modulation . | Strategy . | n . | Population . | Follow-up . | Primary endpoint . | Findings . |
|---|---|---|---|---|---|---|---|
| TOPIC34 | De-escalation | Switch from prasugrel or ticagrelor to clopidogrel | 646 | ACS and PCI | 12 months | NACE | HR, 0.48; 95% CI, 0.34–0.68; P < 0.01 |
| TALOS-AMI35 | De-escalation | Switch from ticagrelor to clopidogrel | 2697 | Prior ACS and PCI | 11 months | NACE | HR, 0.55; 95% CI, 0.40–0.76; P = 0.0001 |
| HOST-REDUCE36 | De-escalation | Dose reduction from prasugrel 10 to 5 mg once daily | 2338 | ACS and PCI | 12 months | NACE | HR, 0.70; 95% CI, 0.52–0.92; P = 0.012 |
| ONE-MONTH DAPT37 | De-escalation | Discontinuation of the P2Y12 inhibitor (1 vs. 6–12 months of DAPT) | 3020 | CCS and PCI | 12 months | NACE | ARD, -0.7%; upper limit of 1-sided 97.5% CI, 1.33%; P < 0.001 for noninferiority |
| SMART-DATE38 | De-escalation | Discontinuation of the P2Y12 inhibitor (6 vs. 12 months of DAPT) | 2712 | ACS and PCI | 18 months | MACE | ARD, 0.5%; upper limit of 1-sided 95% CI, 1.8%; P = 0.03 for noninferiority |
| REDUCE ACS39 | De-escalation | Discontinuation of the P2Y12 inhibitor (3 vs. 12 months of DAPT) | 1496 | ACS and PCI | 12 months | NACE | ARD, -0.0022%; upper limit of 1-sided 95% CI, 0.027%; P < 0.001 for noninferiority |
| DAPT STEMI40 | De-escalation | Discontinuation of the P2Y12 inhibitor (6 vs. 12 months of DAPT) | 1100 | Prior ACS and PCI | 18 months | NACE | HR, 0.73; 95% CI 0.41–1.27; P = 0.004 for noninferiority |
| GLOBAL LEADERS41 | De-escalation | Discontinuation of aspirin (1 vs. 12 months of DAPT) | 15 968 | PCI | 24 months | MACE | Rate ratio, 0.87; 95% CI, 0.75–1.01; P = 0.073) |
| TWILIGHT42 | De-escalation | Discontinuation of aspirin (3 vs. 12 months of DAPT) | 7119 | PCI | 12 months | Bleeding | HR, 0.56; 95% CI, 0.45–0.68; P < 0.001 |
| STOPDAPT-243 | De-escalation | Discontinuation of aspirin (1 vs. 12 months of DAPT) | 3045 | PCI | 12 months | NACE | HR, 0.64; 95% CI, 0.42–0.98; P = 0.04 |
| SMART-CHOICE44 | De-escalation | Discontinuation of aspirin (3 vs. 12 months of DAPT) | 2993 | PCI | 12 months | MACE | ARD, 0.4%, upper limit of 1-sided 95% CI, 1.3%; P = 0.007 for noninferiority |
| TICO45 | De-escalation | Discontinuation of aspirin (3 vs. 12 months of DAPT) | 3056 | ACS | 12 months | NACE | HR, 0.66; 95% CI, 0.48–0.92; P = 0.01 |
| STOPDAPT-2 ACS46 | De-escalation | Discontinuation of aspirin (1–2 vs. 12 months of DAPT) | 4169 | ACS | 12 months | NACE | HR, 1.14; 95% CI, 0.80–1.62; P = 0.06 for noninferiority |
| MASTER DAPT47 | De-escalation | Discontinuation of aspirin or the P2Y12 inhibitor (1 vs. ≥3 months of DAPT) | 4434 | PCI | 11 months | NACE | ARD, -0.23%, upper limit of 95% CI, 1.33%; P < 0.001 for noninferiority |
| HOST-IDEA48 | De-escalation | Discontinuation or the P2Y12 inhibitor (3–6 vs. 12 months of DAPT) | 2013 | PCI | 12 months | NACE | ARD, -0.4%, upper limit of 1-sided 95%, 1.1%; P < 0.001 for noninferiority |
| DAPT49 | Escalation | Add-on P2Y12 inhibitor (30 vs. 12gr months of DAPT) | 9961 | Prior PCI | 18 months | MACE | HR, 0.71; 95% CI, 0.59–0.85; P < 0.001 |
| PEGASUS-TIMI 5450 | Escalation | Add-on ticagrelor (long-term DAPT vs. aspirin) | 21 162 | Prior MI | ∼33 months | MACE | HR (ticagrelor 90 mg), 0.85; 95% CI, 0.75–0.96; P = 0.008; HR (ticagrelor 60 mg), 0.84; 95% CI, 0.74–0.95; P = 0.004 |
| THEMIS51 | Escalation | Add-on ticagrelor (long-term DAPT vs. aspirin) | 19 220 | CCS and diabetes | ∼40 months | MACE | HR, 0.90; 95% CI, 0.81–0.99; P = 0.04 |
Treatment effects are reported for the investigational strategy vs. standard dual antiplatelet therapy. In MASTER DAPT, NACE is reported as the first ranked primary outcome. P-values are for superiority unless otherwise specified. Abbreviations: ACS, acute coronary syndrome; ARD, absolute risk difference; CCS, chronic coronary syndrome; CI, confidence interval; DAPT, dual antiplatelet therapy; HR, hazard ratio; MACE, major adverse cardiac events; MI, myocardial infarction; NACE, net adverse cardiac events; PCI, percutaneous coronary intervention.
Contemporary randomized controlled trials of unselective modulation of antiplatelet therapy
| Trial . | Modulation . | Strategy . | n . | Population . | Follow-up . | Primary endpoint . | Findings . |
|---|---|---|---|---|---|---|---|
| TOPIC34 | De-escalation | Switch from prasugrel or ticagrelor to clopidogrel | 646 | ACS and PCI | 12 months | NACE | HR, 0.48; 95% CI, 0.34–0.68; P < 0.01 |
| TALOS-AMI35 | De-escalation | Switch from ticagrelor to clopidogrel | 2697 | Prior ACS and PCI | 11 months | NACE | HR, 0.55; 95% CI, 0.40–0.76; P = 0.0001 |
| HOST-REDUCE36 | De-escalation | Dose reduction from prasugrel 10 to 5 mg once daily | 2338 | ACS and PCI | 12 months | NACE | HR, 0.70; 95% CI, 0.52–0.92; P = 0.012 |
| ONE-MONTH DAPT37 | De-escalation | Discontinuation of the P2Y12 inhibitor (1 vs. 6–12 months of DAPT) | 3020 | CCS and PCI | 12 months | NACE | ARD, -0.7%; upper limit of 1-sided 97.5% CI, 1.33%; P < 0.001 for noninferiority |
| SMART-DATE38 | De-escalation | Discontinuation of the P2Y12 inhibitor (6 vs. 12 months of DAPT) | 2712 | ACS and PCI | 18 months | MACE | ARD, 0.5%; upper limit of 1-sided 95% CI, 1.8%; P = 0.03 for noninferiority |
| REDUCE ACS39 | De-escalation | Discontinuation of the P2Y12 inhibitor (3 vs. 12 months of DAPT) | 1496 | ACS and PCI | 12 months | NACE | ARD, -0.0022%; upper limit of 1-sided 95% CI, 0.027%; P < 0.001 for noninferiority |
| DAPT STEMI40 | De-escalation | Discontinuation of the P2Y12 inhibitor (6 vs. 12 months of DAPT) | 1100 | Prior ACS and PCI | 18 months | NACE | HR, 0.73; 95% CI 0.41–1.27; P = 0.004 for noninferiority |
| GLOBAL LEADERS41 | De-escalation | Discontinuation of aspirin (1 vs. 12 months of DAPT) | 15 968 | PCI | 24 months | MACE | Rate ratio, 0.87; 95% CI, 0.75–1.01; P = 0.073) |
| TWILIGHT42 | De-escalation | Discontinuation of aspirin (3 vs. 12 months of DAPT) | 7119 | PCI | 12 months | Bleeding | HR, 0.56; 95% CI, 0.45–0.68; P < 0.001 |
| STOPDAPT-243 | De-escalation | Discontinuation of aspirin (1 vs. 12 months of DAPT) | 3045 | PCI | 12 months | NACE | HR, 0.64; 95% CI, 0.42–0.98; P = 0.04 |
| SMART-CHOICE44 | De-escalation | Discontinuation of aspirin (3 vs. 12 months of DAPT) | 2993 | PCI | 12 months | MACE | ARD, 0.4%, upper limit of 1-sided 95% CI, 1.3%; P = 0.007 for noninferiority |
| TICO45 | De-escalation | Discontinuation of aspirin (3 vs. 12 months of DAPT) | 3056 | ACS | 12 months | NACE | HR, 0.66; 95% CI, 0.48–0.92; P = 0.01 |
| STOPDAPT-2 ACS46 | De-escalation | Discontinuation of aspirin (1–2 vs. 12 months of DAPT) | 4169 | ACS | 12 months | NACE | HR, 1.14; 95% CI, 0.80–1.62; P = 0.06 for noninferiority |
| MASTER DAPT47 | De-escalation | Discontinuation of aspirin or the P2Y12 inhibitor (1 vs. ≥3 months of DAPT) | 4434 | PCI | 11 months | NACE | ARD, -0.23%, upper limit of 95% CI, 1.33%; P < 0.001 for noninferiority |
| HOST-IDEA48 | De-escalation | Discontinuation or the P2Y12 inhibitor (3–6 vs. 12 months of DAPT) | 2013 | PCI | 12 months | NACE | ARD, -0.4%, upper limit of 1-sided 95%, 1.1%; P < 0.001 for noninferiority |
| DAPT49 | Escalation | Add-on P2Y12 inhibitor (30 vs. 12gr months of DAPT) | 9961 | Prior PCI | 18 months | MACE | HR, 0.71; 95% CI, 0.59–0.85; P < 0.001 |
| PEGASUS-TIMI 5450 | Escalation | Add-on ticagrelor (long-term DAPT vs. aspirin) | 21 162 | Prior MI | ∼33 months | MACE | HR (ticagrelor 90 mg), 0.85; 95% CI, 0.75–0.96; P = 0.008; HR (ticagrelor 60 mg), 0.84; 95% CI, 0.74–0.95; P = 0.004 |
| THEMIS51 | Escalation | Add-on ticagrelor (long-term DAPT vs. aspirin) | 19 220 | CCS and diabetes | ∼40 months | MACE | HR, 0.90; 95% CI, 0.81–0.99; P = 0.04 |
| Trial . | Modulation . | Strategy . | n . | Population . | Follow-up . | Primary endpoint . | Findings . |
|---|---|---|---|---|---|---|---|
| TOPIC34 | De-escalation | Switch from prasugrel or ticagrelor to clopidogrel | 646 | ACS and PCI | 12 months | NACE | HR, 0.48; 95% CI, 0.34–0.68; P < 0.01 |
| TALOS-AMI35 | De-escalation | Switch from ticagrelor to clopidogrel | 2697 | Prior ACS and PCI | 11 months | NACE | HR, 0.55; 95% CI, 0.40–0.76; P = 0.0001 |
| HOST-REDUCE36 | De-escalation | Dose reduction from prasugrel 10 to 5 mg once daily | 2338 | ACS and PCI | 12 months | NACE | HR, 0.70; 95% CI, 0.52–0.92; P = 0.012 |
| ONE-MONTH DAPT37 | De-escalation | Discontinuation of the P2Y12 inhibitor (1 vs. 6–12 months of DAPT) | 3020 | CCS and PCI | 12 months | NACE | ARD, -0.7%; upper limit of 1-sided 97.5% CI, 1.33%; P < 0.001 for noninferiority |
| SMART-DATE38 | De-escalation | Discontinuation of the P2Y12 inhibitor (6 vs. 12 months of DAPT) | 2712 | ACS and PCI | 18 months | MACE | ARD, 0.5%; upper limit of 1-sided 95% CI, 1.8%; P = 0.03 for noninferiority |
| REDUCE ACS39 | De-escalation | Discontinuation of the P2Y12 inhibitor (3 vs. 12 months of DAPT) | 1496 | ACS and PCI | 12 months | NACE | ARD, -0.0022%; upper limit of 1-sided 95% CI, 0.027%; P < 0.001 for noninferiority |
| DAPT STEMI40 | De-escalation | Discontinuation of the P2Y12 inhibitor (6 vs. 12 months of DAPT) | 1100 | Prior ACS and PCI | 18 months | NACE | HR, 0.73; 95% CI 0.41–1.27; P = 0.004 for noninferiority |
| GLOBAL LEADERS41 | De-escalation | Discontinuation of aspirin (1 vs. 12 months of DAPT) | 15 968 | PCI | 24 months | MACE | Rate ratio, 0.87; 95% CI, 0.75–1.01; P = 0.073) |
| TWILIGHT42 | De-escalation | Discontinuation of aspirin (3 vs. 12 months of DAPT) | 7119 | PCI | 12 months | Bleeding | HR, 0.56; 95% CI, 0.45–0.68; P < 0.001 |
| STOPDAPT-243 | De-escalation | Discontinuation of aspirin (1 vs. 12 months of DAPT) | 3045 | PCI | 12 months | NACE | HR, 0.64; 95% CI, 0.42–0.98; P = 0.04 |
| SMART-CHOICE44 | De-escalation | Discontinuation of aspirin (3 vs. 12 months of DAPT) | 2993 | PCI | 12 months | MACE | ARD, 0.4%, upper limit of 1-sided 95% CI, 1.3%; P = 0.007 for noninferiority |
| TICO45 | De-escalation | Discontinuation of aspirin (3 vs. 12 months of DAPT) | 3056 | ACS | 12 months | NACE | HR, 0.66; 95% CI, 0.48–0.92; P = 0.01 |
| STOPDAPT-2 ACS46 | De-escalation | Discontinuation of aspirin (1–2 vs. 12 months of DAPT) | 4169 | ACS | 12 months | NACE | HR, 1.14; 95% CI, 0.80–1.62; P = 0.06 for noninferiority |
| MASTER DAPT47 | De-escalation | Discontinuation of aspirin or the P2Y12 inhibitor (1 vs. ≥3 months of DAPT) | 4434 | PCI | 11 months | NACE | ARD, -0.23%, upper limit of 95% CI, 1.33%; P < 0.001 for noninferiority |
| HOST-IDEA48 | De-escalation | Discontinuation or the P2Y12 inhibitor (3–6 vs. 12 months of DAPT) | 2013 | PCI | 12 months | NACE | ARD, -0.4%, upper limit of 1-sided 95%, 1.1%; P < 0.001 for noninferiority |
| DAPT49 | Escalation | Add-on P2Y12 inhibitor (30 vs. 12gr months of DAPT) | 9961 | Prior PCI | 18 months | MACE | HR, 0.71; 95% CI, 0.59–0.85; P < 0.001 |
| PEGASUS-TIMI 5450 | Escalation | Add-on ticagrelor (long-term DAPT vs. aspirin) | 21 162 | Prior MI | ∼33 months | MACE | HR (ticagrelor 90 mg), 0.85; 95% CI, 0.75–0.96; P = 0.008; HR (ticagrelor 60 mg), 0.84; 95% CI, 0.74–0.95; P = 0.004 |
| THEMIS51 | Escalation | Add-on ticagrelor (long-term DAPT vs. aspirin) | 19 220 | CCS and diabetes | ∼40 months | MACE | HR, 0.90; 95% CI, 0.81–0.99; P = 0.04 |
Treatment effects are reported for the investigational strategy vs. standard dual antiplatelet therapy. In MASTER DAPT, NACE is reported as the first ranked primary outcome. P-values are for superiority unless otherwise specified. Abbreviations: ACS, acute coronary syndrome; ARD, absolute risk difference; CCS, chronic coronary syndrome; CI, confidence interval; DAPT, dual antiplatelet therapy; HR, hazard ratio; MACE, major adverse cardiac events; MI, myocardial infarction; NACE, net adverse cardiac events; PCI, percutaneous coronary intervention.
De-escalation—By reducing the intensity of platelet inhibition, de-escalation is an approach aimed at decreasing the risk of bleeding complications when they are deemed to be greater than the risk of thrombotic complications. This can be achieved by adjusting the type, dose, or number of antiplatelet drugs used. Several trials have investigated these approaches in contemporary populations mostly including patients undergoing PCI. Importantly, some of these trials only randomized patients who did not experience a thrombotic or bleeding during the initial period of DAPT.35,42,47 This may have resulted in some degree of patient selection and therefore less generalizability compared with trials that randomized at the time of PCI. On the other hand, this design avoided the potential confounding arising from events occurring at that time when the two arms were on the same regimen.
A first common form of de-escalation is switching (i.e. adjusting drug type approach), where a clinician changes from an agent associated with more potent to less potent P2Y12 inhibition.52 This is often performed in patients who have been initially treated for an ACS with a guideline recommended DAPT regimen including prasugrel or ticagrelor, as these drugs are associated with an increased risk of major spontaneous bleeding compared to clopidogrel.53,54 Two trials in ACS, named TOPIC and TALOS-AMI, have demonstrated the net benefit of an unguided switching to clopidogrel at 1 month post-ACS.34,35 Both trials reported a reduction in bleeding as the driving factor behind the improved outcomes.
Another approach to de-escalating antiplatelet therapy is reducing the dose (i.e. adjusting drug dose approach) of the P2Y12 inhibitor characterized by enhanced platelet inhibitory effects (i.e. prasugrel or ticagrelor) used in DAPT combinations. This strategy may be desirable when there are concerns about excessive drug exposure, particularly in ethnicities such as East Asian patients. One trial, the HOST-REDUCE, found that reducing the maintenance dose of prasugrel from 10 to 5 mg once daily at 1 month reduced the risk of net adverse cardiac events (NACE) at 1 year in patients with ACS undergoing PCI.36 Also, the recommended maintenance dose of ticagrelor is 90 mg twice daily for the first year after an ACS but de-escalation to a dosing regimen of 60 mg twice daily can be used after 1 year based on the results of the PEGASUS-TIMI 54 trial, where the 60 mg dosing regimen was found to have similar efficacy to the 90 mg dosing regimen but had a more favorable safety profile in terms of bleeding and non-bleeding side effects.50
A third viable de-escalation approach is the discontinuation of DAPT (i.e. adjusting drug number approach), which means stopping of one of the DAPT antiplatelet agents and transitioning to SAPT with aspirin or a P2Y12 inhibitor. The timing of discontinuation has progressively been moved earlier after PCI with the current generation of coronary stents. Several single-arm studies using historical controls or objective performance goals support the concept that 1 to 3 months of DAPT may be enough in patients at high risk of bleeding.47,55–57 Regardless of bleeding risk, studies such as the ONE-MONTH DAPT trial37,58 in patients with CCS or ACS, and the SMART-DATE trial,38 REDUCE-ACS,39 and DAPT-STEMI40 trials in patients with ACS have shown that early discontinuation of the P2Y12 inhibitor with transition from DAPT to aspirin monotherapy (i.e. at 1 to 6 months) is noninferior to discontinuation at 6 to 12 months in terms of 1-year NACE. These trials used wide noninferiority margins, which raises a note of caution over generalizing their results. Additionally, there were signals of an increase in thrombotic complications with shorter DAPT especially in ACS.38,39,58
Several studies compared strategies of aspirin discontinuation and P2Y12 inhibitor monotherapy after a short (e.g. 1–3 months) period of DAPT in patients undergoing PCI, including GLOBAL LEADERS41 and TWILIGHT42 (testing SAPT with ticagrelor), and STOPDAPT-243 and SMART-CHOICE44 (testing SAPT with clopidogrel). With the exception of the GLOBAL LEADERS that did not meet its primary objective, these studies have shown a reduction in bleeding without any trade-off in ischemic events with P2Y12 inhibitor monotherapy compared with standard DAPT. Two more trials, named TICO45 and STOPDAPT-2 ACS,46 compared early aspirin discontinuation and maintaining SAPT with a P2Y12 inhibitor vs. 12-month DAPT on 1-year NACE specifically in ACS patients: TICO (testing SAPT with ticagrelor) showed superiority, while STOPDAPT-2 ACS (testing SAPT with clopidogrel) failed in showing noninferiority. Although PFT or genetic testing were not performed in STOPDAPT-2 ACS, it may be argued that its findings could be in part attributed to impaired response to clopidogrel which more commonly occurs in high-risk settings. Indeed, this represents an opportunity for future studies of individualized treatment options in which defining response to therapy when relying on SAPT with P2Y12 inhibitor monotherapy should be considered. Of note, ticagrelor monotherapy after a short period of DAPT showed promising results also in ACS sub-analyses of the GLOBAL LEADERS59 and TWILIGHT60 trials.
Finally, some trials compared standard DAPT with strategies of aspirin discontinuation or P2Y12 inhibitor monotherapy at the physician’s discretion. The MASTER DAPT trial selectively randomized patients at high bleeding risk and found that discontinuing the P2Y12 inhibitor or aspirin at 1 month was noninferior to continuing for at least two additional months with respect to 1-year NACE or major adverse cardiac events (MACE), and was associated with reduced bleeding.61 In the HOST-IDEA trial, the discontinuation of one of antiplatelet agent at 3–6 months was noninferior with respect to 1-year NACE in patients with CCS or ACS undergoing PCI.48
Escalation—Antiplatelet therapy escalation is meant to decrease thrombotic or ischemic complications by increasing the intensity of platelet inhibition at a time when such risk is considered greater than the risk of bleeding complications. Again, this can be achieved by adjusting the type, dose, or number of antiplatelet drugs used.
Escalation by switching refers to the practice of changing from a platelet P2Y12 receptor inhibitor with moderate platelet inhibitory effects, such as clopidogrel, to one with stronger platelet inhibitory effects, such as prasugrel or ticagrelor.52 This change is sometimes considered for patients treated with a clopidogrel-based DAPT regimen who present with an acute cardiac event or patients with CCS undergoing high-risk PCI. However, there are currently no data to support that prasugrel or ticagrelor are more effective than clopidogrel among patients undergoing elective PCI.62,63
Escalation by dose increase refers to the practice of increasing the dose of an antiplatelet drug with the goal of intensifying the platelet inhibitory effect. This approach has been tested in a clinical trial of doubled clopidogrel or aspirin dose for patients undergoing PCI64 and a trial of doubled aspirin dose for patients with cardiovascular disease,65 but in both cases, the results have been neutral.
‘Add-on’ escalation refers to the practice of adding a second antiplatelet drug to a patient who is already receiving SAPT, most commonly aspirin. This strategy results in DAPT, and has been shown to be superior to SAPT in reducing ischemic events in various patient populations, including those with a history of prior PCI from the DAPT trial,49 those with prior MI from the PEGASUS-TIMI 54 trial,50 and those with CAD but without a prior acute cardiovascular event and with diabetes mellitus from the THEMIS trial.51 The benefit of DAPT in THEMIS was more pronounced in patients with prior PCI.66
Platelet function and genotype-guided approaches
Despite compliance to antiplatelet treatment, some patients may still experience ischemic events, a phenomenon known as ‘therapeutic failure’.67 A number of factors contribute to therapeutic failure, including impaired response to an antiplatelet agent, commonly referred to as ‘drug resistance’. Such finding is supported by a number of observational studies, mostly conducted in patients undergoing PCI and treated with clopidogrel-based DAPT, in which the use of PFT has shown that individuals who persist with high on-treatment platelet reactivity (HPR) are at increased risk for thrombotic complications.68 Some studies have also shown that patients with low on-treatment platelet reactivity are at increased risk of bleeding,68 although the overall evidence seems more robust for the prediction of ischemic risk.
The efficacy of clopidogrel is dependent on a two-step activation process by the hepatic cytochrome P450 (CYP) system, with the CYP2C19 enzyme involved in both metabolic steps.9,69 Notably, CYP2C19 is highly polymorphic, meaning that variants in the gene confer wide variability in the enzyme’s metabolic activity across individuals. Therefore, there is wide variability in the activation of clopidogrel and in the resulting platelet inhibitory effects across individuals. Prasugrel and ticagrelor, however, are not affected by such genetic polymorphisms resulting in more consistent and sustained platelet inhibitory effects.9 Carriers of CYP2C19 loss of function (LOF) alleles have significantly reduced clopidogrel metabolism, resulting in lower levels of active metabolite, reduced clopidogrel-induced platelet inhibition and higher rates of thrombotic complications in patients undergoing PCI.70,71 The most common LOF alleles include CYP2C19*2 and CYP2C19*3; less common LOF alleles include CYP2C19 *4, *5, *6, *7, and *8. On the contrary, CYP2C19*17 is considered a gain of function allele and is associated with increased transcription and enzyme expression.9 Combinations of CYP2C19 alleles define the metabolizer status and serve for the basis of therapeutic recommendations (Table 4). 72 Allele frequencies vary by ancestry, with the highest prevalences among of East Asians.73
Clopidogrel therapy based on CYP2C19 phenotype for ACS/PCI patients initiating antiplatelet therapy
| Phenotype (genotype) . | Implications for clopidogrel . | Therapeutic recommendations . |
|---|---|---|
| UM (*1/*17, *17/*17) and EM (*1/*1) | Normal (EM) or increased (UM) platelet inhibition; normal (EM) or decreased (UM) residual platelet aggregation | Clopidogrel label-recommended dosage and administration |
| Intermediate metabolizers (*1/*2) | Reduced platelet inhibition; increased residual platelet aggregation; increased risk for adverse cardiovascular events | Prasugrel or ticagrelor (if no contraindications) |
| PM (*2/*2) | Significantly reduced platelet inhibition; increased residual platelet aggregation; increased risk for adverse cardiovascular events | Prasugrel or ticagrelor (if no contraindications) |
| Phenotype (genotype) . | Implications for clopidogrel . | Therapeutic recommendations . |
|---|---|---|
| UM (*1/*17, *17/*17) and EM (*1/*1) | Normal (EM) or increased (UM) platelet inhibition; normal (EM) or decreased (UM) residual platelet aggregation | Clopidogrel label-recommended dosage and administration |
| Intermediate metabolizers (*1/*2) | Reduced platelet inhibition; increased residual platelet aggregation; increased risk for adverse cardiovascular events | Prasugrel or ticagrelor (if no contraindications) |
| PM (*2/*2) | Significantly reduced platelet inhibition; increased residual platelet aggregation; increased risk for adverse cardiovascular events | Prasugrel or ticagrelor (if no contraindications) |
Adapted from Scott et al.72 Abbreviations: EM, extensive metabolizers; PM, poor metabolizers; UM, ultrarapid metabolizers.
Clopidogrel therapy based on CYP2C19 phenotype for ACS/PCI patients initiating antiplatelet therapy
| Phenotype (genotype) . | Implications for clopidogrel . | Therapeutic recommendations . |
|---|---|---|
| UM (*1/*17, *17/*17) and EM (*1/*1) | Normal (EM) or increased (UM) platelet inhibition; normal (EM) or decreased (UM) residual platelet aggregation | Clopidogrel label-recommended dosage and administration |
| Intermediate metabolizers (*1/*2) | Reduced platelet inhibition; increased residual platelet aggregation; increased risk for adverse cardiovascular events | Prasugrel or ticagrelor (if no contraindications) |
| PM (*2/*2) | Significantly reduced platelet inhibition; increased residual platelet aggregation; increased risk for adverse cardiovascular events | Prasugrel or ticagrelor (if no contraindications) |
| Phenotype (genotype) . | Implications for clopidogrel . | Therapeutic recommendations . |
|---|---|---|
| UM (*1/*17, *17/*17) and EM (*1/*1) | Normal (EM) or increased (UM) platelet inhibition; normal (EM) or decreased (UM) residual platelet aggregation | Clopidogrel label-recommended dosage and administration |
| Intermediate metabolizers (*1/*2) | Reduced platelet inhibition; increased residual platelet aggregation; increased risk for adverse cardiovascular events | Prasugrel or ticagrelor (if no contraindications) |
| PM (*2/*2) | Significantly reduced platelet inhibition; increased residual platelet aggregation; increased risk for adverse cardiovascular events | Prasugrel or ticagrelor (if no contraindications) |
Adapted from Scott et al.72 Abbreviations: EM, extensive metabolizers; PM, poor metabolizers; UM, ultrarapid metabolizers.
Several studies have tried to answer whether a strategy of tailoring antiplatelet therapy with guidance from PFT or genotyping is associated with clinical benefit (Table 5). A meta-analysis including 11 randomised controlled trials and three observational studies of guided selection of antiplatelet therapy (PFT and genetic testing) for patients undergoing PCI (n = 20 743 patients) showed improvements in both composite and individual efficacy outcomes.83 More specifically, outcomes varied according to the strategy used, with an escalation approach associated with a significant reduction in ischaemic events without any trade-off in safety, and a de-escalation approach by switch or dose reduction associated with a significant reduction in bleeding, without any trade-off in efficacy. In a more recent network meta-analysis of 15 randomised controlled trials in patients with an ACS (n = 61 898 patients), compared with routine selection of potent P2Y12 inhibiting therapy (prasugrel or ticagrelor), a guided selection of P2Y12 inhibiting therapy was found to be associated with the most favourable balance between safety and efficacy.84
Strategy trials of modulation of antiplatelet therapy guided by platelet function or genetic testing in patients undergoing percutaneous coronary intervention
| Trial . | Intervention . | n . | Population . | Follow-up . | Primary endpoint . | Findings . |
|---|---|---|---|---|---|---|
| Trials of PFT guidance | ||||||
| ARCTIC74 | PFT-guided escalation or de-escalation by switch or dose adjustment | 2240 | CCS or ACS | 12 months | MACE | HR, 1.13; 95% CI, 0.98–1.29; P = 0.10 |
| ANTARCTIC75 | PFT-guided escalation or de-escalation by switch or dose adjustment | 877 | ACS | 12 months | NACE | HR, 1.00, 95% CI 0.78–1.29; P = 0.98 |
| TROPICAL ACS76 | PFT-guided de-escalation by switch | 2610 | ACS | 12 months | NACE | HR, 0.81; 95% CI, 0.62–1.06; P = 0.0004 for noninferiority |
| PATH-PCI77 | PFT-guided escalation by switch | 2237 | CCS | 6 months | NACE | HR, 0.68, 95% CI, 0.49–0.95; P = 0.023 |
| Trials of genetic guidance | ||||||
| PHARMCLO78 | Genotype-guided de-escalation by switch | 888 | ACS | 12 months | NACE | HR, 0.58; 95% CI, 0.43–0.78; P < 0.001 |
| POPULAR GENETICS79 | Genotype-guided de-escalation by switch | 2488 | ACS | 12 months | NACE | ARD, -0.7%; upper limit of the 95% CI, 0.7%; P < 0.001 for noninferiority |
| ADAPT-PCI80 | Genotype-guided escalation by switch | 509 | CCS or ACS | ∼16 months | Use of prasugrel or ticagrelor | OR, 1.60; 95% CI, 1.07–2.42; P = 0.03 |
| TAILOR-PCI81 | Genotype-guided escalation by switch | 1849 | CCS or ACS | 12 months | MACE | HR, 0.66; 95% CI, 0.43–1.02; P = 0.06 |
| Al-Rubaish et al.82 | Genotype-guided escalation by switch | 755 | ACS | 12 months | NACE | OR, 0.34; 95% CI, 0.20–0.59; P = NA |
| Trial . | Intervention . | n . | Population . | Follow-up . | Primary endpoint . | Findings . |
|---|---|---|---|---|---|---|
| Trials of PFT guidance | ||||||
| ARCTIC74 | PFT-guided escalation or de-escalation by switch or dose adjustment | 2240 | CCS or ACS | 12 months | MACE | HR, 1.13; 95% CI, 0.98–1.29; P = 0.10 |
| ANTARCTIC75 | PFT-guided escalation or de-escalation by switch or dose adjustment | 877 | ACS | 12 months | NACE | HR, 1.00, 95% CI 0.78–1.29; P = 0.98 |
| TROPICAL ACS76 | PFT-guided de-escalation by switch | 2610 | ACS | 12 months | NACE | HR, 0.81; 95% CI, 0.62–1.06; P = 0.0004 for noninferiority |
| PATH-PCI77 | PFT-guided escalation by switch | 2237 | CCS | 6 months | NACE | HR, 0.68, 95% CI, 0.49–0.95; P = 0.023 |
| Trials of genetic guidance | ||||||
| PHARMCLO78 | Genotype-guided de-escalation by switch | 888 | ACS | 12 months | NACE | HR, 0.58; 95% CI, 0.43–0.78; P < 0.001 |
| POPULAR GENETICS79 | Genotype-guided de-escalation by switch | 2488 | ACS | 12 months | NACE | ARD, -0.7%; upper limit of the 95% CI, 0.7%; P < 0.001 for noninferiority |
| ADAPT-PCI80 | Genotype-guided escalation by switch | 509 | CCS or ACS | ∼16 months | Use of prasugrel or ticagrelor | OR, 1.60; 95% CI, 1.07–2.42; P = 0.03 |
| TAILOR-PCI81 | Genotype-guided escalation by switch | 1849 | CCS or ACS | 12 months | MACE | HR, 0.66; 95% CI, 0.43–1.02; P = 0.06 |
| Al-Rubaish et al.82 | Genotype-guided escalation by switch | 755 | ACS | 12 months | NACE | OR, 0.34; 95% CI, 0.20–0.59; P = NA |
Treatment effects are reported for the investigational strategy vs. standard dual antiplatelet therapy; P-values are for superiority unless otherwise specified. Abbreviations: ACS; acute coronary syndrome; ARD, absolute risk difference; CI, confidence interval; HPR, high platelet reactivity; HR, hazard ratio; LOF, loss of function; MACE; major adverse cardiac events; NA, not available; NACE, net adverse cardiac events; OR, odds ratio; PCI, percutaneous coronary intervention; PFT, platelet function testing.
Strategy trials of modulation of antiplatelet therapy guided by platelet function or genetic testing in patients undergoing percutaneous coronary intervention
| Trial . | Intervention . | n . | Population . | Follow-up . | Primary endpoint . | Findings . |
|---|---|---|---|---|---|---|
| Trials of PFT guidance | ||||||
| ARCTIC74 | PFT-guided escalation or de-escalation by switch or dose adjustment | 2240 | CCS or ACS | 12 months | MACE | HR, 1.13; 95% CI, 0.98–1.29; P = 0.10 |
| ANTARCTIC75 | PFT-guided escalation or de-escalation by switch or dose adjustment | 877 | ACS | 12 months | NACE | HR, 1.00, 95% CI 0.78–1.29; P = 0.98 |
| TROPICAL ACS76 | PFT-guided de-escalation by switch | 2610 | ACS | 12 months | NACE | HR, 0.81; 95% CI, 0.62–1.06; P = 0.0004 for noninferiority |
| PATH-PCI77 | PFT-guided escalation by switch | 2237 | CCS | 6 months | NACE | HR, 0.68, 95% CI, 0.49–0.95; P = 0.023 |
| Trials of genetic guidance | ||||||
| PHARMCLO78 | Genotype-guided de-escalation by switch | 888 | ACS | 12 months | NACE | HR, 0.58; 95% CI, 0.43–0.78; P < 0.001 |
| POPULAR GENETICS79 | Genotype-guided de-escalation by switch | 2488 | ACS | 12 months | NACE | ARD, -0.7%; upper limit of the 95% CI, 0.7%; P < 0.001 for noninferiority |
| ADAPT-PCI80 | Genotype-guided escalation by switch | 509 | CCS or ACS | ∼16 months | Use of prasugrel or ticagrelor | OR, 1.60; 95% CI, 1.07–2.42; P = 0.03 |
| TAILOR-PCI81 | Genotype-guided escalation by switch | 1849 | CCS or ACS | 12 months | MACE | HR, 0.66; 95% CI, 0.43–1.02; P = 0.06 |
| Al-Rubaish et al.82 | Genotype-guided escalation by switch | 755 | ACS | 12 months | NACE | OR, 0.34; 95% CI, 0.20–0.59; P = NA |
| Trial . | Intervention . | n . | Population . | Follow-up . | Primary endpoint . | Findings . |
|---|---|---|---|---|---|---|
| Trials of PFT guidance | ||||||
| ARCTIC74 | PFT-guided escalation or de-escalation by switch or dose adjustment | 2240 | CCS or ACS | 12 months | MACE | HR, 1.13; 95% CI, 0.98–1.29; P = 0.10 |
| ANTARCTIC75 | PFT-guided escalation or de-escalation by switch or dose adjustment | 877 | ACS | 12 months | NACE | HR, 1.00, 95% CI 0.78–1.29; P = 0.98 |
| TROPICAL ACS76 | PFT-guided de-escalation by switch | 2610 | ACS | 12 months | NACE | HR, 0.81; 95% CI, 0.62–1.06; P = 0.0004 for noninferiority |
| PATH-PCI77 | PFT-guided escalation by switch | 2237 | CCS | 6 months | NACE | HR, 0.68, 95% CI, 0.49–0.95; P = 0.023 |
| Trials of genetic guidance | ||||||
| PHARMCLO78 | Genotype-guided de-escalation by switch | 888 | ACS | 12 months | NACE | HR, 0.58; 95% CI, 0.43–0.78; P < 0.001 |
| POPULAR GENETICS79 | Genotype-guided de-escalation by switch | 2488 | ACS | 12 months | NACE | ARD, -0.7%; upper limit of the 95% CI, 0.7%; P < 0.001 for noninferiority |
| ADAPT-PCI80 | Genotype-guided escalation by switch | 509 | CCS or ACS | ∼16 months | Use of prasugrel or ticagrelor | OR, 1.60; 95% CI, 1.07–2.42; P = 0.03 |
| TAILOR-PCI81 | Genotype-guided escalation by switch | 1849 | CCS or ACS | 12 months | MACE | HR, 0.66; 95% CI, 0.43–1.02; P = 0.06 |
| Al-Rubaish et al.82 | Genotype-guided escalation by switch | 755 | ACS | 12 months | NACE | OR, 0.34; 95% CI, 0.20–0.59; P = NA |
Treatment effects are reported for the investigational strategy vs. standard dual antiplatelet therapy; P-values are for superiority unless otherwise specified. Abbreviations: ACS; acute coronary syndrome; ARD, absolute risk difference; CI, confidence interval; HPR, high platelet reactivity; HR, hazard ratio; LOF, loss of function; MACE; major adverse cardiac events; NA, not available; NACE, net adverse cardiac events; OR, odds ratio; PCI, percutaneous coronary intervention; PFT, platelet function testing.
PFT—The availability of user-friendly and bedside assays has enabled the execution of multicentre randomized clinical trials in patients undergoing PCI testing the impact on outcomes associated with the use of PFT. Some of these trials where specifically conducted among patients with HPR (identified by PFT) and comparing different antiplatelet treatments,85,86 while other trials compared PFT guidance used as a strategy vs. standard care.74–77
The GRAVITAS trial included patients with HPR, who were randomly assigned to high-dose clopidogrel or standard-dose clopidogrel; the primary composite outcome showed no significant difference between the groups at 6 months.86 The TRIGGER PCI trial, also conducted selectively in patients with HPR, compared prasugrel and clopidogrel, but was terminated prematurely due to a lower-than-anticipated risk of events at 6 months.85 The ARCTIC and ANTARCTIC studies, evaluating a bedside strategy for monitoring and adjusting antiplatelet therapy (i.e. de-escalation or escalation by switch or dose adjustment as appropriate), also failed to show significant benefits.74,75
The inability of these early trials to demonstrate any clinical benefit with the use of PFT could be attributed to a number of factors, such as inclusion of low-risk patients, limited use of novel generation P2Y12 inhibitors, and inadequate cut-off values to define HPR. More recent studies conducted in high-risk settings have shown more promising findings.76,77 TROPICAL-ACS trial enrolled patients with ACS undergoing PCI, randomly assigned to either PFT guidance with de-escalation by switch from prasugrel to clopidogrel if responders or standard care.76 The trial, powered for noninferiority on NACE vs. standard DAPT, met its primary objective. The PATH-PCI trial also met its primary objective, showing superiority for the PFT-based approach.77
Guidance by genetic testing—Rapid CYP2C19 genotyping assays, with results available within 60 min, are commercially available making genetic testing feasible in real-world practice.87,88 Genetic testing has been evaluated as a method of guiding antiplatelet therapy in five studies of patients with PCI and/or ACS, with mixed results78–82 (Table 5).
In patients with ACS undergoing PCI, the PHARMCLO78 and POPULAR GENETICS79 trials compared the genotype-based selection of P2Y12 inhibitors (i.e. selective use of prasugrel or ticagrelor among CYP2C19 LOF carriers and clopidogrel in noncarriers) to unguided standard of care DAPT. Despite being terminated early and therefore resulting in lower power than anticipated, PHARMCLO showed the genotype-based approach to significantly reduce the risk of NACE. Similarly, in POPULAR GENETICS, the genotype-based approach was non-inferior to standard therapy in terms of NACE at 12 months, and resulted in a significantly lower incidence of major or minor bleedings.
In the ADAPT-PCI trial,80 the genotype-guided prescription was found to significantly increase the use of prasugrel or ticagrelor compared to an unguided approach in patients with ACS or CCS undergoing PCI. TAILOR-PCI81 was the largest trial of genotype-guided selection of oral P2Y12 inhibitor in which patients with ACS or CCS undergoing PCI were randomized to genotype-guidance or conventional therapy. In the genotype-guide group, CYP2C19 LOF carriers were prescribed ticagrelor and noncarriers clopidogrel. Patients randomized to the conventional group were prescribed clopidogrel. Although the genotype-guided approach showed a numerical reduction in MACE, this did not reach statistical significance, possibly due to a lack of power. However, a recent analysis from the TAILOR-PCI study did show superiority of a genotype-guided approach when considering recurrent events.89 Conversely, a trial from Al-Rubaish et al.,82 who only included patients with ACS, showed a significant benefit of the genotype-guided strategy in reducing NACE.
PFT and genetic testing: advantages and disadvantages
PFT has the advantage of being more closely related with the clinical outcome (i.e. increased thrombotic and bleeding complications with high and low platelet reactivity, respectively).16 Nevertheless, results of PFTs are subject to variability, particularly with clopidogrel early after treatment initiation, requiring patients to be on treatment for a certain period of time (e.g. for at least 1–2 weeks) to adequately assess antiplatelet drug response. Hence, the potential need for serial assessments as well as switching between different oral P2Y12 inhibitors may be challenging to implement in clinical practice.90 In contrast, results of genetic testing remain unchanged and allow to determine, even prior to therapy, the CYP2C19 phenotype associated with different degrees of clopidogrel response (Table 4). Accordingly, CYP2C19 LOF carriers can be treated with ticagrelor or prasugrel while noncarriers with clopidogrel. The disadvantage of relying solely on genetic testing is that the pharmacodynamic effects of clopidogrel as measured by PFT depend on multiple factors and not solely on CYP2C19 genotypes.91 To this extent, integrating genetic data with clinical variables, such as in the ABCD-GENE (Age, Body Mass Index, Chronic Kidney Disease, Diabetes, and Genotyping) score has shown to enhance the accuracy in identifying patients with impaired clopidogrel response.92 Although retrospective assessments have shown the ability of the ABCD-GENE score to identify patients at increased ischemic risk, prospective validation of this score is still warranted.93,94
Antiplatelet therapies, personalised strategies under clinical testing, and unmet needs
The challenge for new antiplatelet drugs for CAD is to exhibit less bleeding without reducing antithrombotic potency. Some of these developments may include new formulations of already available drugs, such as a phospholipid-aspirin complex which is designed to reduce gastro-intestinal injury and readily absorbed to ensure antithrombotic efficacy.95 Other drugs include novel experimental agents aimed at new targets. Potential new therapeutics are in advanced preclinical development or have already entered the clinical development phase.96 It remains to be seen how these new agents will fit within current paradigms of antiplatelet therapy and whether they will lead to safer combinations in clinical practice.
The value of risk stratification tools to guide clinical decisions is typically assessed by means of c-statistics indicating their discrimination ability. However, whether a risk score is helpful in improving clinical outcomes requires an intervention-based randomized clinical study. Trials of personalised duration of antiplatelet therapy based on the DAPT score (NCT04135989) and the PRECISE-DAPT score (NCT05732701, NCT03848572) are underway. Several ongoing randomized clinical trials are also addressing the role of PFT-guided strategies of antiplatelet therapy modulation in patients with ACS (NCT04755387, NCT04718025, NCT04240834, NCT04937699, NCT04338919, NCT05262803) and genotype-guided strategies in patients undergoing PCI (NCT03783351).
There are a number of unmet needs that may be stimulating for future studies. In fact, de-escalation strategies have used DAPT as a comparator and there is limited data comparing the different approaches. For example, there are no direct comparisons of de-escalation strategies involving aspirin withdrawal (i.e. P2Y12 inhibitor monotherapy) vs. switch guided by PFT or genotype testing. Additionally, most of the trials and evidence cited in this review investigated clinical outcomes over a relatively short time interval (i.e. 1 to 3 years). While strategies for long-term secondary prevention are also evolving rapidly, it is unclear which patients may be ideally suited for long-term DAPT vs. dual pathway inhibition the low-dose direct oral anticoagulants (i.e. rivaroxaban) vs. P2Y12 inhibitor monotherapy.
Guidelines
The ESC guidelines for myocardial revascularization97 and those for ACS22 include a low-grade recommendation (i.e. class IIb) for de-escalation by switch of P2Y12 receptor inhibitor treatment (e.g. from prasugrel or ticagrelor to clopidogrel), or de-escalation by dose reduction, as an alternative DAPT strategy ‘especially for ACS patients deemed unsuitable for potent platelet inhibition’. Also, the recommendation states that de-escalation by switch ‘may be done unguided based on clinical judgment or guided by PFT or CYP2C19 genotyping, depending on patient’s risk profile and availability of respective assays’. The level of evidence for this recommendation is A, with the TOPIC, TROPICAL-ACS, and POPULAR GENETICS trials used as supporting references. The phrasing of the recommendation, referring to patients ‘unsuitable for potent platelet inhibition’, implies that these approaches for now are best suited to patients at high bleeding risk, a population that was actually understudied in the abovementioned trials, while the application of a strategy should reflect the types of patients enrolled in the respective trials. No recommendation for de-escalation by switch has been issued by the recent American guidelines for myocardial revascularization.4
The ESC guidelines also include class IIa recommendations for de-escalation by discontinuation of the P2Y12 inhibitor at 3 months (level of evidence B, in patients at high bleeding risk as define by the PRECISE-DAPT score or by the ARC-HBR criteria) or aspirin at 3–6 months (level of evidence A, ‘depending on the balance between the ischemic and bleeding risk’). In the American guidelines, the recommendation for P2Y12 inhibitor discontinuation at 6 months is class 2b (for patients at ‘high bleeding risk or overt bleeding on DAPT’) and the recommendation for aspirin at 1–3 months discontinuation is 2a (potentially for any patient).
Additionally, the ESC guidelines include recommendations for escalation by ‘add-on’ of a P2Y12 inhibitor (class IIa, level of evidence A for patients ‘with a high risk of ischemic events and without increased risk of major or life-threatening bleeding’ and class IIb, level of evidence A for patients ‘with a moderate risk of ischemic events and without increased risk of major or life-threatening bleeding’). In the American guidelines, this approach is recommended with class 2b for patients ‘with no high risk of bleeding and no significant overt bleeding on DAPT’.
Conclusions
Personalised antiplatelet therapy has emerged as a crucial approach in optimizing the balance between efficacy and safety by customizing antiplatelet therapy to individual patient's needs and risk profile. Accurate risk stratification for both bleeding and thrombosis based on multiple factors can aid in selecting the appropriate antiplatelet therapy and prevent serious and life-threatening outcomes. With the introduction of PFT and rapid genotype characterization, personalised antiplatelet therapy is becoming more feasible and accessible. The comprehensive understanding of personalised antiplatelet therapy presented in this review can help clinicians make informed clinical decisions and tailor cardiovascular disease management.
Declarations
Disclosure of Interest
D.C. reports speaker’s or consulting fees from Amgen, Arena, Chiesi, Daiichi Sankyo, Sanofi, Terumo, and institutional fees from Medtronic. D.J.A. declares that he has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, Novartis, PhaseBio, PLx Pharma, Pfizer, Sanofi, and Vectura, outside the present work; D.J.A. also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and Scott R. MacKenzie Foundation.
Data Availability
No new data were generated or analysed for this manuscript.
Funding
The research leading to this review has received funding from the European Union—NextGenerationEU through the Italian Ministry of University and Research under PNRR—M4C2-I1.3 Project PE_00000019 ‘HEAL ITALIA’ to Davide Capodanno, CUP E63C22002080006 of University of Catania. The views and opinions expressed are those of the authors only and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them.



