-
PDF
- Split View
-
Views
-
Cite
Cite
Richard K Cheng, Sarah A M Cuddy, Neurohormonal blockade in transthyretin amyloidosis: perhaps one size does not fit all?, European Heart Journal, Volume 44, Issue 31, 14 August 2023, Pages 2908–2910, https://doi.org/10.1093/eurheartj/ehad357
Close - Share Icon Share
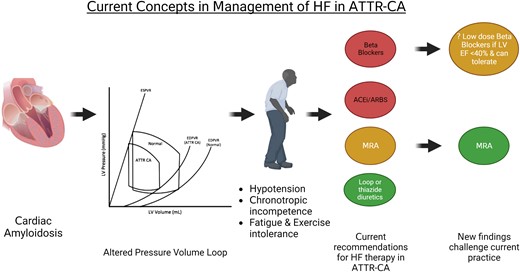
Current concepts in the management of heart failure in ATTR-CA. Myocardial amyloid infiltration alters the pressure–volume loop. The resulting symptoms include hypotension, exercise intolerance, and fatigue; chronotropic incompetence is also a feature of the disease. Patients often poorly tolerate conventional neurohormonal-blocking medications. This has led to both the ESC6 and AHA7 recommending that ACEi/ARB and beta-blockers are avoided (red—avoid) in ATTR-CA. The AHA 2020 scientific statement7 recommended consideration (yellow) of an MRA in conjunction with a loop diuretic (green—recommended). The study of Ioannou et al.2 challenges these recommendations. ACEi/ARB, angiotensin-converting enzyme inhibitors/angiotensin receptor blocker; ATTR-CA, transthyretin cardiac amyloidosis; MRA, mineralocorticoid receptor antagonist.
This editorial refers to ‘Conventional heart failure therapy in cardiac ATTR amyloidosis’, by A. Ioannou et al., https://doi.org/10.1093/eurheartj/ehad347.
Historically thought of as a rare disease, transthyretin (ATTR) cardiac amyloidosis (CA) is increasingly diagnosed due to improved awareness and advances in non-invasive imaging techniques. Many cases of ATTR-CA are now diagnosed at earlier stages of disease, with less pathological remodelling of their cardiac chambers, which has previously been demonstrated.1 However, a proportion of these patients still present with more advanced disease, reflected by significant left ventricular (LV) dysfunction and a reduced ejection fraction (LVEF) ≤ 40% (22.4% of patients in the study by Ioannou et al., published in this issue of the European Heart Journal2). Given the increased prevalence of ATTR-CA, it is critically important to understand if conventional heart failure (HF) medications may be of benefit.
Patients with ATTR-CA appear to respond differently to neurohormonal (NH) blockade with angiotensin-converting enzyme inhibitors (ACEis)/angiotensin receptor blockers (ARBs), beta-blockers, and mineralocorticoid receptor antagonists (MRAs), as compared with other patients with HF. This is attributed to poor haemodynamic tolerance of these therapies because of an altered pressure–volume loop; stroke volume is relatively fixed and there is potential ventricular–vascular decoupling.3 Afterload reduction may predispose to hypotension without augmenting stroke volume, and a reduction of heart rate may negatively impair cardiac output given the fixed stroke volume, as well as blunting the chronotropic response needed to augment cardiac output during exercise.4 In light of these considerations and clinical experience, recent consensus statements and guidelines recommend the use of NH blockade with caution and avoidance of beta-blockers in ATTR-CA5–8 despite lack of robust data supporting these recommendations (Graphical Abstract). Small studies have evaluated NH blockade in ATTR-CA with inconsistent findings.9–11 The current retrospective study2 aims to fill this knowledge gap in ATTR-CA; it provides incremental data from a large cohort in an attempt to further address the effect of NH blockade in patients with ATTR-CA.
The current study2 has many strengths that build on the relative scarcity of data in this space. It includes the largest cohort to date consisting of 2371 patients with ATTR-CA exposed to NH blockade. The authors have longitudinal data on medication use, prescription patterns, dosages, discontinuation rates, and association with hard clinical endpoints. There was a large proportion (22.4%) of patients with variant ATTR-CA (ATTRv); this is unique as many previously published cohorts consisted predominantly of wild-type ATTR-CA.
In the study of Ioannou et al., 71.9% were New York Heart Association (NYHA) functional class I–II, with a mean LVEF of 48.2%, and 81.8% were National Amyloidosis Centre (NAC) stage 1 (45.8%) or 2 (36.0%). For NH blockade use, 55.4% of patients were treated with beta-blockers, 57.4% with ACEis/ARBs, and 39% with MRAs. For beta-blockers, the majority (88.7%) were on bisoprolol at low doses, ≤2.5 mg per day, 63.1% were prescribed ≤25% of the target dose, and only 5.7% reached the recommended target dose for HF. The use of device therapy in the current study was relatively low. In the total cohort, only 9.0% had pacemakers and for those with LVEF ≤40%, only 3.2% had defibrillators.2
The most intriguing aspect of the study of Ioannou et al. was the finding that beta-blockers confer benefit in ATTR-CA with reduced LVEF.2 In the total cohort, there was no difference in mortality between those on beta-blockers and those not on beta-blockers. Conversely, a pre-specified analysis of 338 patients with LVEF ≤40% found a lower mortality in those on beta-blockers [hazard ratio (HR) 0.61, 95% confidence interval (CI) 0.45–0.83]. While there was a signal of benefit for those treated with beta-blockers, there was no signal of harm when stopping beta-blockers (during follow-up, 21.7% had beta-blockers discontinued and these patients were characterized by lower blood pressure and heart rate). The study raises multiple intriguing questions. The authors did not find a dose effect,2 which is unexpected if the benefit is predominantly from NH blockade. Notably, patients in the study were on low doses of beta-blockers. In other HF cohorts, up-titration of medications will typically result in a more robust treatment effect. Hence, is it possible that in ATTR-CA patients, the association of beta-blocker dose and benefit is non-linear and U-shaped? In other words, perhaps at low doses the NH blockade properties are beneficial, but is it possible that the negative haemodynamic on-treatment effects may be detrimental at higher doses? Further, in the current study, 88.7% of the patients were on bisoprolol.2 There is regional variation in beta-blocker medication use; for example, in the USA, the majority of patients with HF with reduced LVEF are on carvedilol or metoprolol succinate, with bisoprolol typically reserved for patients intolerant of other beta-blockers. Is it possible that in ATTR-CA there are differences in tolerance and effect among the various beta-blockers?
In contrast to the current study,2 another recently published study did not demonstrate a benefit of NH blockade use in ATTR-CA and suggested that discontinuation of beta-blockers may improve outcomes.9 There were some key differences in the patient cohorts that may explain these apparently discordant findings that are imperative to highlight. There were baseline differences in the patients, with those in the prior study being sicker (41.7% NYHA functional class III, included patients with neuropathy, mean systolic blood pressure of 115 mmHg, and 30.1% had a pacemaker)9 compared with the current study (18.3% NYHA functional class III, excluded those with neuropathy, and mean systolic blood pressure of 125 mmHg).2 Further, almost all patients in the prior study were on carvedilol or metoprolol (only one patient was on bisoprolol) and the median dosing was higher (10 mg/day carvedilol, equivalent to bisoprolol 5 mg/day).9 Use of beta-blockers in ATTR-CA needs to be considered on an individual case basis. Many unknowns remain; whether patients with more advanced disease may be less tolerant; whether the treatment effect is consistent across the different beta-blockers; and whether optimal dosing of beta-blockers in ATTR-CA may be lower than in other HF cohorts.
The findings with ACEis/ARBs and MRAs were mostly consistent with prior literature. For ACEi/ARB use, there was no benefit in either the total cohort or for those with LVEF ≤40%; consistent with prior studies.9,10 Lastly, for MRA use, the authors found a survival benefit in the total cohort with MRA use (HR 0.77, 95% CI 0.66–0.89). This is in line with a retrospective analysis of the TOPCAT trial in a subcohort enriched for echocardiographic characteristics consistent with cardiac amyloidosis.12
To put these effect sizes into perspective, the reported relative risk reduction with beta-blockers and MRAs is similar to what was demonstrated in the ATTR-ACT study with tafamidis as compared with placebo (HR 0.70, 95% CI 0.51–0.96) for all-cause mortality,13 but at a much lower medication cost. However, retrospective data must always be interpreted cautiously. This has previously been seen in amyloidosis; doxycycline was assumed to confer a survival benefit based on retrospective data.14 This was disproven when tested prospectively.15 Despite the robust analytical approach that incorporated sensitivity analyses, there is an inability to account for residual confounding in a non-randomized study and potential for treatment bias. These forms of confounding can be particularly difficult to fully account for in pharmaco-epidemiology. For example, it is possible that clinical providers may have avoided NH blockade use in the sickest patients with hypotension or frailty. Very few patients (only 55) were started on HF medications after establishing care at the NAC, and most were already on their respective HF regimens. Hence, there may be pre-existing treatment bias of stopping HF medications in intolerant patients before the initial visit at the NAC.
In order to fully answer the question of NH blockade use in ATTR-CA, we need to consider what we can do to move the field forward. Most importantly, this serves as a call to action regarding the need for a randomized clinical trial of NH blockade in patients with ATTR-CA. Benefit may not be a ‘one size fits all’ and we will need to be selective about who we treat. The current study suggests that benefit is most likely in those with LVEF ≤40% with beta-blockers and in all-comers with MRAs; additionally, if the benefit is from NH blockade, perhaps there will be a signal of benefit with low-dose ACEis/ARBs. In the interim, we recommend consideration of pragmatic trials with close collaboration between amyloid centres of expertise worldwide in an effort to expand the sample size and include a more diverse cohort. Such collaborations will be needed not only to evaluate conventional HF therapies, but also to assess more novel therapies that may have potential benefit in ATTR-CA, such as sodium–glucose co-transporter 2 (SGLT2) inhibitors, and address the unmet needs in the management of CA.
Declarations
Disclosure of Interest
None declared.
Funding
Dr Cuddy is supported by the National Institutes of Health grants K23 HL166686 and the American Heart Association 23CDA857664.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.



