-
PDF
- Split View
-
Views
-
Cite
Cite
Moritz F Sinner, Aenne S von Falkenhausen, A specific new biomarker for atrial fibrillation and its sequelae?, European Heart Journal, Volume 44, Issue 3, 14 January 2023, Pages 219–220, https://doi.org/10.1093/eurheartj/ehac645
Close - Share Icon Share
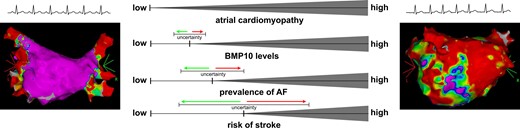
Atrial cardiomyopathy continuously develops over the life span. A healthy (left) and a severely altered (right) atrium are symbolized by electro-anatomical mapping results and by the electrocardiogram rhythm strips in sinus rhythm and atrial fibrillation, respectively. In relation to the progressing atrial cardiomyopathy, bone morphogenetic protein 10 (BMP10) levels, the prevalence of atrial fibrillation, and the risk of stroke also increase. Yet, the exact magnitude and timing of their associations have not been fully determined, as indicated by the increasing degree of uncertainty visualized by the error bars. Electro-anatomical mapping images obtained from the authors' own repositories, with permissions.
This editorial refers to ‘Bone morphogenetic protein 10: a novel risk marker of ischaemic stroke in patients with atrial fibrillation’, by Z. Hijazi et al., https://doi.org/10.1093/eurheartj/ehac632.
Atrial fibrillation (AF) is undeniably an important healthcare challenge. AF is worsening functional status due to symptoms; AF is deteriorating health by worsening underlying conditions including heart failure; and AF is predisposing to novel conditions such as cardio-embolic stroke and impaired cognitive function. Treatment options exist. Rate and rhythm control strategies include antiarrhythmic drugs and catheter ablation to alleviate symptoms and improve health outcomes. Furthermore, easily applicable scores such as the CHA2DS2-VASc score estimate the risk of stroke in the context of AF and guide antithrombotic therapies.1
Yet, all existing options for AF treatment remain suboptimal. Both drug-based and catheter-based rhythm control strategies retain relevant recurrence rates of AF.1 Despite guideline-recommended anticoagulation, patients are left with a residual stroke rate.1 In many instances, it is not sufficiently possible to predict such treatment failure. These shortcomings may in part be explained by our often unspecific characterization of AF. AF is an electrocardiogram-based diagnosis, which is mostly categorized by its time course and clinical burden of symptoms.1
AF can be characterized in more detail, but most measures are either non-specific or tedious to determine. Non-specific predisposing measures may include age, sex, and the profile of comorbidities. Measures more difficult to determine encompass echocardiographic left atrial size, atrial strain, flow velocity in the left atrial appendage, magnetic resonance imaging-derived atrial fibrosis, or electro-anatomical mapping-based atrial low voltage areas.2 Beyond that, laboratory biomarkers are useful to characterize and predict AF, namely N-terminal pro B-type natriuretic protein (NT-proBNP) and troponin T.3,4 NT-proBNP is an established marker of atrial stretch, whereas troponin T is elevated in response to cardiomyocyte damage. As such, both laboratory biomarkers are non-specific for AF.
Bone morphogenetic protein 10 (BMP10) has the potential to be a more specific marker of AF. In this issue of the European Heart Journal, Dr Hijazi and colleagues add important novel insights on BMP10 to the field.5 The authors conducted a large and comprehensive analysis in patients with AF to associate BMP10 with the occurrence of stroke. To achieve this goal, the authors analysed the participants of three large and well-known randomized clinical trials which differ by their antithrombotic treatment regimens. All participants presented with a diagnosis of AF at baseline and were treated either with aspirin in the ACTIVE A and AVERROES trials or with warfarin or apixaban in the ARISTOTLE trial. The incidence of stroke differed markedly between patients receiving antiplatelet treatment or anticoagulation. Yet, in both groups, BMP10 levels were similar in magnitude and were highly significantly associated with the occurrence of stroke. The relationship remained significant even when accounting for the established predictors of AF or stroke, including NT-proBNP. Interestingly, BMP10 also seemed to improve prediction of stroke and appeared to be specifically associated with stroke in the context of AF. It improved the model’s performance beyond the information conveyed by NT-proBNP. Also, unlike NT-proBNP, it did not show a relevant association with other important outcomes such as major bleeding, death, and heart failure.5
Taken together, the authors of this current publication sharpen the profile of a novel, measurable laboratory biomarker, BMP10, which has the potential to improve our understanding of AF and its consequences. It may thereby guide our treatment of this common and relevant arrhythmia. Yet, important aspects related to this novel marker are not discussed or touched upon in this publication. Why is there a residual risk of stroke in AF patients, even when placed on sufficient anticoagulation by current standards? Is BMP10 truly a marker of AF, or is BMP10 solely associated with AF as a proxy?
Most probably, these questions cannot be answered fully yet. However, we submit that the more recent concept of an atrial cardiomyopathy may hypothetically provide an explanation as illustrated in our Graphical Abstract. The temporal relationship of strokes and the detection of AF episodes in the ASSERT trial revealed that such AF episodes do not necessarily immediately precede cerebral insults.6 Instead, AF episodes can either occur remotely prior to the stroke, or the stroke can even precede detection of AF. If AF episodes immediately preceding a stroke were not missed, then other factors could have predisposed to thrombus formation. Such factors could be atrial structural, electrical, and mechanical alterations resembling the atrial cardiomyopathy.7 As in most tissues, age, diseases, and environmental factors also induce and promote alterations in the atria as an increasing continuum.
It is so far unclear if BMP10 mirrors the full trajectory of atrial cardiomyopathy. In the present investigation, the authors support that BMP10 levels are indeed increasing with age and the actual presence and burden of AF at blood draw,5 factors that are also compatible with the presence and aggravation of an atrial cardiomyopathy. Yet, the BMP10 levels in healthy individuals or the general population remain to be determined. Also, the interaction between AF and BMP10 requires further investigation. The results of the current and prior work support a clear association between the two. Exemplarily, BMP10 levels were associated with the recurrence of AF after a cardioversion,8 with the recurrence of AF after catheter ablation for AF,9 and with the detection of AF in patients suffering from a cryptogenic stroke.10 The causality of these associations is unclear. Yet again, it appears plausible that BMP10 levels reflect a more severely altered atrial structure, which reduces the effectiveness of rhythm control therapies, or which facilitates thrombogenesis preceding cardio-embolism. It is important to note that the interpretation of the relationship between an atrial cardiomyopathy with BMP10 levels, with the prevalence of AF, and with the risk of stroke is affected by increasing levels of uncertainty (Graphical Abstract).
The understanding of BMP10 as a marker of atrial cardiomyopathy is also supported by experimental work. BMP10 levels in the blood depend on the expression of the gene PITX2.9PITX2 is located on chromosome 4 adjacent to the strongest genome-wide association signal for AF.11 In early work, PITX2 has been identified as a transcription factor important for cardiac left–right asymmetry and the development of the pulmonary veins during mouse embryogenesis.12 Given the importance of the pulmonary veins for catheter ablation of AF, a possible causal relationship between AF and the PITX2 gene product BMP10 appeared plausible. More recent work, however, clarified that PITX2 modulates a gene network to maintain atrial rhythm by regulating atrial ion channel and structural proteins.13 It may be such processes that underlie the electrical and structural remodelling observed as atrial cardiomyopathy in AF patients.
In summary, the new insights into AF and stroke pathophysiology presented by Hijazi et al. are highly valuable. The improved characterization of BMP10 as an easily measurable biomarker of high atrial specificity might improve clinical applicability in the near future. However, the causal role of BMP10 in the pathophysiology of AF or atrial cardiomyopathy, respectively, will require further elaboration.
Data availability
No novel data are associated with this article.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
Conflict of interest: None declared.



